Characterization of APOBEC3G binding to 7SL RNA
- PMID: 18597676
- PMCID: PMC2494554
- DOI: 10.1186/1742-4690-5-54
Characterization of APOBEC3G binding to 7SL RNA
Abstract
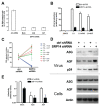
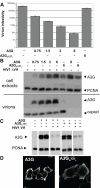
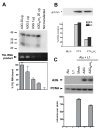
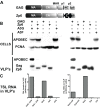
Human APOBEC3 proteins are editing enzymes that can interfere with the replication of exogenous retroviruses such as human immunodeficiency virus (HIV), hepadnaviruses such as hepatitis B virus (HBV), and with the retrotransposition of endogenous retroelements such as long-interspersed nuclear elements (LINE) and Alu. Here, we show that APOBEC3G, but not other APOBEC3 family members, binds 7SL RNA, the common ancestor of Alu RNAs that is specifically recruited into HIV virions. Our data further indicate that APOBEC3G recognizes 7SL RNA and Alu RNA by its common structure, the Alu domain, suggesting a mechanism for APOBEC3G- mediated inhibition of Alu retrotransposition. However, we also demonstrate that APOBEC3F and APOBEC3G are normally recruited into and inhibit the infectivity of DeltaVif HIV1 virions when 7SLRNA is prevented from accessing particles by RNA interference against SRP14 or by over expression of SRP19, both components of the signal recognition particle. We thus conclude that 7SL RNA is not an essential mediator of the virion packaging of these antiviral cytidine deaminases.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

