A novel and exploitable antifungal peptide from kale (Brassica alboglabra) seeds
- PMID: 18597893
- PMCID: PMC7115674
- DOI: 10.1016/j.peptides.2008.05.020
A novel and exploitable antifungal peptide from kale (Brassica alboglabra) seeds
Abstract
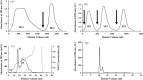
The aim of this study was to purify and characterize antifungal peptides from kale seeds in view of the paucity of information on antifungal peptides from the family Brassicaceae, and to compare its characteristics with those of published Brassica antifungal peptides. A 5907-Da antifungal peptide was isolated from kale seeds. The isolation procedure comprised affinity chromatography on Affi-gel blue gel, ion exchange chromatography on SP-Sepharose and Mono S, and gel filtration on Superdex Peptide. The peptide was adsorbed on the first three chromatographic media. It inhibited mycelial growth in a number of fungal species including Fusarium oxysporum, Helminthosporium maydis, Mycosphaerella arachidicola and Valsa mali, with an IC(50) of 4.3microM, 2.1microM, 2.4microM, and 0.15microM, respectively and exhibited pronounced thermostability and pH stability. It inhibited proliferation of hepatoma (HepG2) and breast cancer (MCF7) cells with an IC(50) of 2.7microM and 3.4microM, and the activity of HIV-1 reverse transcriptase with an IC(50) of 4.9microM. Its N-terminal sequence differed from those of antifungal proteins which have been reported to date.
Figures




References
-
- Broekaert W.F., Marien W., Terras F.R., De Bolle M.F., Proost P., Van Damme J. Antimicrobial peptides from Amaranthus caudatus seeds with sequence homology to the cysteine/glycine-rich domain of chitin-binding proteins. Biochemistry. 1992;31:4308–4314. - PubMed
-
- Broekaert W.F., Van Parijs J., Leyns F., Joos H., Peumans W.J. A chitin-binding lectin from stinging nettle rhizomes with antifungal properties. Science. 1989;245:1100–1102. - PubMed
-
- Bulet P., Hetru C., Dimarcq J.L., Hoffmann D. Antimicrobial peptides in insects; structure and function. Dev Comp Immunol. 1999;23:329–344. - PubMed
-
- Ceciliani F., Bortolotti F., Menegatti E., Ronchi S., Ascenzi P., Palmieri S. Purification, inhibitory properties, amino acid sequence and identification of the reactive site of a new serine proteinase inhibitor from oil-rape (Brassica napus) seed. FEBS Lett. 1994;342:221–224. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

