Synchrony between orientation-selective neurons is modulated during adaptation-induced plasticity in cat visual cortex
- PMID: 18598368
- PMCID: PMC2481260
- DOI: 10.1186/1471-2202-9-60
Synchrony between orientation-selective neurons is modulated during adaptation-induced plasticity in cat visual cortex
Abstract
Background: Visual neurons respond essentially to luminance variations occurring within their receptive fields. In primary visual cortex, each neuron is a filter for stimulus features such as orientation, motion direction and velocity, with the appropriate combination of features eliciting maximal firing rate. Temporal correlation of spike trains was proposed as a potential code for linking the neuronal responses evoked by various features of a same object. In the present study, synchrony strength was measured between cells following an adaptation protocol (prolonged exposure to a non-preferred stimulus) which induce plasticity of neurons' orientation preference.
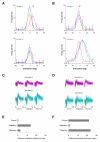
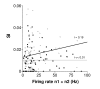
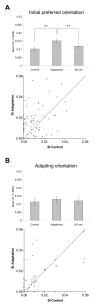
Results: Multi-unit activity from area 17 of anesthetized adult cats was recorded. Single cells were sorted out and (1) orientation tuning curves were measured before and following 12 min adaptation and 60 min after adaptation (2) pairwise synchrony was measured by an index that was normalized in relation to the cells' firing rate. We first observed that the prolonged presentation of a non-preferred stimulus produces attractive (58%) and repulsive (42%) shifts of cell's tuning curves. It follows that the adaptation-induced plasticity leads to changes in preferred orientation difference, i.e. increase or decrease in tuning properties between neurons. We report here that, after adaptation, the neuron pairs that shared closer tuning properties display a significant increase of synchronization. Recovery from adaptation was accompanied by a return to the initial synchrony level.
Conclusion: We conclude that synchrony reflects the similarity in neurons' response properties, and varies accordingly when these properties change.
Figures





Similar articles
-
Visual cells remember earlier applied target: plasticity of orientation selectivity.PLoS One. 2008;3(11):e3689. doi: 10.1371/journal.pone.0003689. Epub 2008 Nov 10. PLoS One. 2008. PMID: 18997867 Free PMC article.
-
Adaptation-induced plasticity and spike waveforms in cat visual cortex.Neuroreport. 2012 Jan 25;23(2):88-92. doi: 10.1097/WNR.0b013e32834e7e71. Neuroreport. 2012. PMID: 22143199
-
Repetitive adaptation induces plasticity of spatial frequency tuning in cat primary visual cortex.Neuroscience. 2011 Jan 13;172:355-65. doi: 10.1016/j.neuroscience.2010.10.017. Epub 2010 Oct 20. Neuroscience. 2011. PMID: 20969932
-
Adaptation-induced plasticity in the sensory cortex.J Neurophysiol. 2022 Oct 1;128(4):946-962. doi: 10.1152/jn.00114.2022. Epub 2022 Sep 21. J Neurophysiol. 2022. PMID: 36130163 Review.
-
Bottom-up and top-down dynamics in visual cortex.Prog Brain Res. 2005;149:65-81. doi: 10.1016/S0079-6123(05)49006-8. Prog Brain Res. 2005. PMID: 16226577 Review.
Cited by
-
The effect of neural adaptation on population coding accuracy.J Comput Neurosci. 2012 Jun;32(3):387-402. doi: 10.1007/s10827-011-0358-4. Epub 2011 Sep 14. J Comput Neurosci. 2012. PMID: 21915690 Free PMC article.
-
Beyond Rehabilitation of Acuity, Ocular Alignment, and Binocularity in Infantile Strabismus.Front Syst Neurosci. 2018 Jul 18;12:29. doi: 10.3389/fnsys.2018.00029. eCollection 2018. Front Syst Neurosci. 2018. PMID: 30072876 Free PMC article.
-
Visual adaptation to convexity in macaque area V4.Neuroscience. 2009 Jun 30;161(2):655-62. doi: 10.1016/j.neuroscience.2009.03.070. Epub 2009 Apr 5. Neuroscience. 2009. PMID: 19345725 Free PMC article.
-
Adaptation of cerebral oxygen metabolism and blood flow and modulation of neurovascular coupling with prolonged stimulation in human visual cortex.Neuroimage. 2013 Nov 15;82:182-9. doi: 10.1016/j.neuroimage.2013.05.110. Epub 2013 May 31. Neuroimage. 2013. PMID: 23732885 Free PMC article.
-
Visual cells remember earlier applied target: plasticity of orientation selectivity.PLoS One. 2008;3(11):e3689. doi: 10.1371/journal.pone.0003689. Epub 2008 Nov 10. PLoS One. 2008. PMID: 18997867 Free PMC article.
References
-
- Bishop PO, Henry GH. Striate neurons: receptive field concepts. Invest Ophthalmol. 1972;11:346–354. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

