Longitudinal selenium status in healthy British adults: assessment using biochemical and molecular biomarkers
- PMID: 18598587
- PMCID: PMC3137460
- DOI: 10.1017/S0007114508006831
Longitudinal selenium status in healthy British adults: assessment using biochemical and molecular biomarkers
Abstract
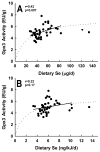
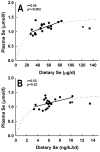
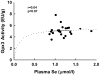
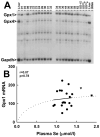
Human selenium (Se) requirements are currently based on biochemical markers of Se status. In rats, tissue glutathione peroxidase-1 (Gpx1) mRNA levels can be used effectively to determine Se requirements; blood Gpx1 mRNA levels decrease in Se-deficient rats, so molecular biology-based markers have potential for human nutrition assessment. To study the efficacy of molecular biology markers for assessing Se status in humans, we conducted a longitudinal study on 39 subjects (age 45 +/- 11) in Reading, UK. Diet diaries (5 day) and blood were obtained from each subject at 2, 8, 17 and 23 weeks, and plasma Se, glutathione peroxidase (Gpx3) enzyme activity, and selenoprotein mRNA levels were determined. There were no significant longitudinal effects on Se biomarkers. Se intake averaged 48 +/- 14 microg/d. Plasma Se concentrations averaged 1.13 +/- 0.16 micromol/l. Plasma Se v. energy-corrected Se intake (ng Se/kJ/d) was significantly correlated, but neither Gpx3 activity v. Se intake (ng Se/kJ/d) nor Gpx3 activity v. plasma Se was significantly correlated. Collectively, this indicates that subjects were on the plateaus of the response curves. Selenoprotein mRNAs were quantitated in total RNA isolated from whole blood, but mRNA levels for Gpx1, selenoprotein H, and selenoprotein W (all highly regulated by Se in rodents), as well selenoprotein P, Gpx3, and phospholipid hydroperoxide glutathione peroxidase were also not significantly correlated with plasma Se. Thus selenoprotein molecular biomarkers, as well as traditional biochemical markers, are unable to further distinguish differences in Se status in these Se replete subjects. The efficacy of molecular biomarkers to detect Se deficiency needs to be tested in Se-deficient populations.
Figures
Similar articles
-
Genetic variants in selenoprotein genes modulate biomarkers of selenium status in response to Brazil nut supplementation (the SU.BRA.NUT study).Clin Nutr. 2019 Apr;38(2):539-548. doi: 10.1016/j.clnu.2018.03.011. Epub 2018 Mar 23. Clin Nutr. 2019. PMID: 29609868
-
Blood glutathione peroxidase-1 mRNA levels can be used as molecular biomarkers to determine dietary selenium requirements in rats.Exp Biol Med (Maywood). 2009 Nov;234(11):1271-9. doi: 10.3181/0906-RM-182. Exp Biol Med (Maywood). 2009. PMID: 19855070
-
Impact of Glutathione Peroxidase-1 (Gpx1) Genotype on Selenoenzyme and Transcript Expression When Repleting Selenium-Deficient Mice.Biol Trace Elem Res. 2018 Nov;186(1):174-184. doi: 10.1007/s12011-018-1281-6. Epub 2018 Mar 3. Biol Trace Elem Res. 2018. PMID: 29502249
-
Insights for Setting of Nutrient Requirements, Gleaned by Comparison of Selenium Status Biomarkers in Turkeys and Chickens versus Rats, Mice, and Lambs.Adv Nutr. 2016 Nov 15;7(6):1129-1138. doi: 10.3945/an.116.012872. Print 2016 Nov. Adv Nutr. 2016. PMID: 28140330 Free PMC article. Review.
-
Selenium, selenoproteins and human health: a review.Public Health Nutr. 2001 Apr;4(2B):593-9. doi: 10.1079/phn2001143. Public Health Nutr. 2001. PMID: 11683552 Review.
Cited by
-
Effects of selenium supplementation on selenoprotein gene expression and response to influenza vaccine challenge: a randomised controlled trial.PLoS One. 2011 Mar 21;6(3):e14771. doi: 10.1371/journal.pone.0014771. PLoS One. 2011. PMID: 21445287 Free PMC article. Clinical Trial.
-
Critical issues in setting micronutrient recommendations for pregnant women: an insight.Matern Child Nutr. 2010 Oct;6 Suppl 2(Suppl 2):5-22. doi: 10.1111/j.1740-8709.2010.00269.x. Matern Child Nutr. 2010. PMID: 22296248 Free PMC article. Review.
-
Selenium regulation of the selenoprotein and nonselenoprotein transcriptomes in rodents.Adv Nutr. 2011 Mar;2(2):138-50. doi: 10.3945/an.110.000240. Epub 2011 Mar 10. Adv Nutr. 2011. PMID: 22332043 Free PMC article. Review.
-
Establishing optimal selenium status: results of a randomized, double-blind, placebo-controlled trial.Am J Clin Nutr. 2010 Apr;91(4):923-31. doi: 10.3945/ajcn.2009.28169. Epub 2010 Feb 24. Am J Clin Nutr. 2010. PMID: 20181815 Free PMC article. Clinical Trial.
-
DNA damage and oxidative stress response to selenium yeast in the non-smoking individuals: a short-term supplementation trial with respect to GPX1 and SEPP1 polymorphism.Eur J Nutr. 2016 Dec;55(8):2469-2484. doi: 10.1007/s00394-015-1118-4. Epub 2015 Dec 10. Eur J Nutr. 2016. PMID: 26658762 Free PMC article.
References
-
- Clark LC, Combs GF, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. JAMA. 1996;276:1957–1963. - PubMed
-
- Broadley MR, White PJ, Bryson RJ, et al. Biofortification of UK food crops with selenium. Proc Nutr Soc. 2006;65:169–181. - PubMed
-
- Rayman MP. The importance of selenium to human health. Lancet. 2000;356:233–241. - PubMed
-
- Elsom R, Sanderson P, Hesketh JE, Jackson MJ, Fairweather-Tait SJ, Akesson B, Handy J, Arthur JR. Functional markers of selenium status: UK Foods Standards Agency workshop report. Br J Nutr. 2006;96:980–984. - PubMed
-
- Rayman MP. Food-chain selenium and human health: emphasis on intake. Br J Nutr. 2008:1–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

