Transmission of Bartonella henselae by Ixodes ricinus
- PMID: 18598628
- PMCID: PMC2600320
- DOI: 10.3201/eid1407.071110
Transmission of Bartonella henselae by Ixodes ricinus
Abstract
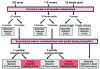
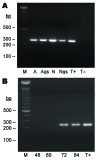
Bartonella spp. are facultative intracellular bacteria associated with several emerging diseases in humans and animals. B. henselae causes cat-scratch disease and is increasingly associated with several other syndromes, particularly ocular infections and endocarditis. Cats are the main reservoir for B. henselae and the bacteria are transmitted to cats by cat fleas. However, new potential vectors are suspected of transmitting B. henselae, in particular, Ixodes ricinus, the most abundant ixodid tick that bites humans in western Europe. We used a membrane-feeding technique to infect I. ricinus with B. henselae and demonstrate transmission of B. henselae within I. ricinus across developmental stages, migration or multiplication of B. henselae in salivary glands after a second meal, and transmission of viable and infective B. henselae from ticks to blood. These results provide evidence that I. ricinus is a competent vector for B. henselae.
Figures

References
-
- Marsilia GM, La Mura A, Galdiero R, Galdiero E, Aloj G, Ragozzino A. Isolated hepatic involvement of cat scratch disease in immunocompetent adults: enhanced magnetic resonance imaging, pathological findings, and molecular analysis–two cases. Int J Surg Pathol. 2006;14:349–54. 10.1177/1066896906291780 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
