Age-related alterations in oxidatively damaged proteins of mouse skeletal muscle mitochondrial electron transport chain complexes
- PMID: 18598756
- PMCID: PMC2873767
- DOI: 10.1016/j.freeradbiomed.2008.06.006
Age-related alterations in oxidatively damaged proteins of mouse skeletal muscle mitochondrial electron transport chain complexes
Abstract
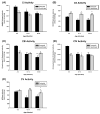
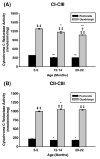
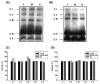
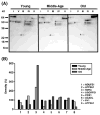
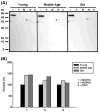
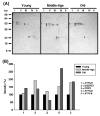
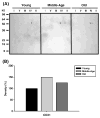
Age-associated mitochondrial dysfunction is a major source of reactive oxygen species (ROS) and oxidative modification to proteins. Mitochondrial electron transport chain (ETC) complexes I and III are the sites of ROS production and we hypothesize that proteins of the ETC complexes are primary targets of ROS-mediated modification which impairs their structure and function. The pectoralis, primarily an aerobic red muscle, and quadriceps, primarily an anaerobic white muscle, have different rates of respiration and oxygen-carrying capacity, and hence, different rates of ROS production. This raises the question of whether these muscles exhibit different levels of oxidative protein modification. Our studies reveal that the pectoralis shows a dramatic age-related decline in almost all complex activities that correlates with increased oxidative modification. Similar complex proteins were modified in the quadriceps, at a significantly lower level with less change in enzyme and ETC coupling function. We postulate that mitochondrial ROS causes damage to specific ETC subunits which increases with age and leads to further mitochondrial dysfunction. We conclude that physiological characteristics of the pectoralis vs quadriceps may play a role in age-associated rate of mitochondrial dysfunction and in the decline in tissue function.
Figures




References
-
- Roubenoff R. Sarcopenia: a major modifiable cause of frailty in the elderly. J Nutr Health Aging. 2000;4:140–142. - PubMed
-
- Marzetti E, Leeuwenburgh C. Skeletal muscle apoptosis, sarcopenia and frailty at old age. Exp Gerontol. 2006;41:1234–1238. - PubMed
-
- Kamel HK. Sarcopenia and aging. Nutr Rev. 2003;61:157–167. - PubMed
-
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

