Biosynthesis, degradation and pharmacological importance of the fatty acid amides
- PMID: 18598910
- PMCID: PMC2667908
- DOI: 10.1016/j.drudis.2008.02.006
Biosynthesis, degradation and pharmacological importance of the fatty acid amides
Abstract
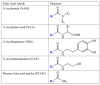
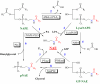
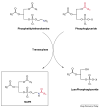
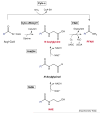
The identification of two biologically active fatty acid amides, N-arachidonoylethanolamine (anandamide) and oleamide, has generated a great deal of excitement and stimulated considerable research. However, anandamide and oleamide are merely the best-known and best-understood members of a much larger family of biologically occurring fatty acid amides. In this review, we will outline which fatty acid amides have been isolated from mammalian sources, detail what is known about how these molecules are made and degraded in vivo, and highlight their potential for the development of novel therapeutics.
Figures
References
-
- Thudichum JLW. Chemical Constitution of the Brain. Archon Books; 1962.
-
- Levene PA. Sphingomyelin. III. J. Biol. Chem. 1916;24:69–89.
-
- Kuehl KA, Jr., et al. The identification of N-(2-hydroxyethyl)-palmitamide as a naturally occurring anti-inflammatory agent. J. Amer. Chem Soc. 1957;79:5577–5578.
-
- Devane WA, et al. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258(5090):1946–1949. - PubMed
-
- Koga D, et al. Liquid chromatographic-atmospheric pressure chemical ionization mass spectrometric determination of anandamide and its analogs in rat brain and peripheral tissues. J Chromatogr B Biomed Sci Appl. 1997;690(12):7–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

