Reentry in survived subepicardium coupled to depolarized and inexcitable midmyocardium: insights into arrhythmogenesis in ischemia phase 1B
- PMID: 18598961
- PMCID: PMC2821335
- DOI: 10.1016/j.hrthm.2008.03.025
Reentry in survived subepicardium coupled to depolarized and inexcitable midmyocardium: insights into arrhythmogenesis in ischemia phase 1B
Abstract
Background: Delayed ventricular arrhythmias during acute myocardial ischemia (1B arrhythmias) are associated with an increase in tissue impedance and are most likely sustained in a thin subepicardial layer.
Objective: The goal of this study was to test the hypothesis that heterogeneous uncoupling between depolarized midmyocardium and surviving subepicardium results in heterogeneous refractoriness in the latter, providing the reentry substrate after a premature beat.
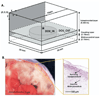
Methods: A 3-dimensional bidomain slab was constructed comprising a normal subepicardial layer coupled to a slightly depolarized (-80 to -60 mV) but inexcitable midmyocardium. Experimentally measured tissue impedance served as input for the model. Four stages of heterogeneous uncoupling between the 2 layers were simulated, each corresponding to an experimental ischemic impedance value. Effective refractory periods (ERP), conduction velocities, and inducibility of reentry were examined.
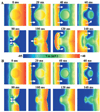
Results: Heterogeneous uncoupling resulted in subepicardial ERP dispersion, allowing reentry to occur. The minimum ERP dispersion needed to induce reentry was 28 ms. Reentry induction was only possible in this model at the 2 intermediate stages of uncoupling, and only when midmyocardial resting membrane potential was more negative than -60 mV. Complete uncoupling of the layers resulted in normal subepicardial conduction without arrhythmias. The minimum length of the reentrant pathway was 2.5 cm, comparable to 2.4 cm reported in previous experiments.
Conclusion: Heterogeneous uncoupling to a negative sink such as depressed inexcitable midmyocardium may be a substrate for ischemia 1B arrhythmias. Total uncoupling removes the arrhythmogenic substrate.
Figures





Comment in
-
Progress in modeling cardiac electrical activity: a long way from spherical cows.Heart Rhythm. 2008 Jul;5(7):1045-6. doi: 10.1016/j.hrthm.2008.04.006. Epub 2008 Apr 12. Heart Rhythm. 2008. PMID: 18598962 No abstract available.
Similar articles
-
Mechanisms for initiation of reentry in acute regional ischemia phase 1B.Heart Rhythm. 2010 Mar;7(3):379-86. doi: 10.1016/j.hrthm.2009.11.014. Epub 2009 Nov 13. Heart Rhythm. 2010. PMID: 20097623 Free PMC article.
-
Role of cellular uncoupling in arrhythmogenesis in ischemia phase 1B.Conf Proc IEEE Eng Med Biol Soc. 2006;2006:2272-5. doi: 10.1109/IEMBS.2006.259470. Conf Proc IEEE Eng Med Biol Soc. 2006. PMID: 17945702
-
Late ventricular arrhythmias during acute regional ischemia in the isolated blood perfused pig heart. Role of electrical cellular coupling.Cardiovasc Res. 2001 May;50(2):362-72. doi: 10.1016/s0008-6363(01)00222-x. Cardiovasc Res. 2001. PMID: 11334840
-
Generation of arrhythmias in myocardial ischemia and infarction.Am J Cardiol. 1988 Jan 15;61(2):20A-26A. doi: 10.1016/0002-9149(88)90737-0. Am J Cardiol. 1988. PMID: 2447770 Review.
-
Myocardial ischemia: what factors determine arrhythmogenesis?J Cardiovasc Electrophysiol. 2001 Jun;12(6):726-9. doi: 10.1046/j.1540-8167.2001.00726.x. J Cardiovasc Electrophysiol. 2001. PMID: 11405409 Review.
Cited by
-
Mechanisms for initiation of reentry in acute regional ischemia phase 1B.Heart Rhythm. 2010 Mar;7(3):379-86. doi: 10.1016/j.hrthm.2009.11.014. Epub 2009 Nov 13. Heart Rhythm. 2010. PMID: 20097623 Free PMC article.
-
Mechanisms Underlying the Emergence of Post-acidosis Arrhythmia at the Tissue Level: A Theoretical Study.Front Physiol. 2017 Mar 30;8:195. doi: 10.3389/fphys.2017.00195. eCollection 2017. Front Physiol. 2017. PMID: 28424631 Free PMC article.
-
Exploring susceptibility to atrial and ventricular arrhythmias resulting from remodeling of the passive electrical properties in the heart: a simulation approach.Front Physiol. 2014 Nov 12;5:435. doi: 10.3389/fphys.2014.00435. eCollection 2014. Front Physiol. 2014. PMID: 25429272 Free PMC article. Review.
-
Advances in modeling ventricular arrhythmias: from mechanisms to the clinic.Wiley Interdiscip Rev Syst Biol Med. 2014 Mar-Apr;6(2):209-24. doi: 10.1002/wsbm.1256. Epub 2013 Dec 6. Wiley Interdiscip Rev Syst Biol Med. 2014. PMID: 24375958 Free PMC article. Review.
-
Computational rabbit models to investigate the initiation, perpetuation, and termination of ventricular arrhythmia.Prog Biophys Mol Biol. 2016 Jul;121(2):185-94. doi: 10.1016/j.pbiomolbio.2016.06.004. Epub 2016 Jun 19. Prog Biophys Mol Biol. 2016. PMID: 27334789 Free PMC article. Review.
References
-
- Kaplinsky E, Ogawa S, Balke CW, et al. Two periods of early ventricular arrhythmia in the canine acute myocardial infarction model. Circulation. 1979;60:397–403. - PubMed
-
- de Groot JR, Wilms-Schopman FJ, Opthof T, et al. Late ventricular arrhythmias during acute regional ischemia in the isolated blood perfused pig heart. Role of electrical cellular coupling. Cardiovasc Res. 2001;50:362–372. - PubMed
-
- Janse MJ, van Capelle FJ, Morsink H, et al. Flow of “injury” current and patterns of excitation during early ventricular arrhythmias in acute regional myocardial ischemia in isolated porcine and canine hearts. Evidence for two different arrhythmogenic mechanisms. Circ Res. 1980;47:151–165. - PubMed
-
- Pogwizd SM, Corr PB. Reentrant and nonreentrant mechanisms contribute to arrhythmogenesis during early myocardial ischemia: results using three-dimensional mapping. Circ Res. 1987;61:352–371. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

