Steatosis reversibly increases hepatocyte sensitivity to hypoxia-reoxygenation injury
- PMID: 18599084
- PMCID: PMC2784014
- DOI: 10.1016/j.jss.2007.12.784
Steatosis reversibly increases hepatocyte sensitivity to hypoxia-reoxygenation injury
Abstract
Background: Steatosis decreases survival of liver grafts after transplantation due to poorly understood mechanisms. We examined the effect of steatosis on the survival of liver grafts in a rat liver transplantation model and the viability of cultured rat hepatocytes after hypoxia and reoxygenation.
Materials and methods: Rats were fed a choline and methionine-deficient diet to induce hepatic steatosis, and the livers were transplanted into recipient rats after 6 h of cold storage. Cultured hepatocytes were made steatotic by incubation for 3 d in fatty acid-supplemented medium. Hypoxia and reoxygenation were induced by placing the cultures in a 90% N(2)/10% CO(2) atmosphere for 4 h, followed by return to normoxic conditions for 6 h. Hepatocyte viability was assessed by lactate dehydrogenase release and mitochondrial potential staining.
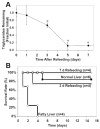
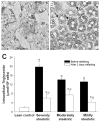
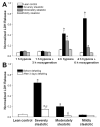
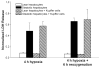
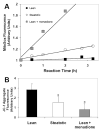
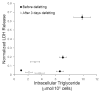
Results: Transplanted steatotic livers exhibited 0% viability compared with 90% for lean liver controls. When donor choline and methionine-deficient diet rats were returned to a normal diet, hepatic fat content decreased while viability of the grafts after transplantation increased. Cultured steatotic hepatocytes generated more mitochondrial superoxide, exhibited a lowered mitochondrial membrane potential, and released significantly more lactate dehydrogenase after hypoxia and reoxygenation than lean hepatocyte controls. When steatotic hepatocytes were defatted by incubating in fatty acid-free medium, they became less sensitive to hypoxia and reoxygenation as the remaining intracellular triglyceride content decreased.
Conclusions: Hepatic steatosis reversibly decreases viability of hepatocytes after hypoxia and reoxygenation in vitro. The decreased viability of steatotic livers after transplantation may be due to a direct effect of hypoxia and reoxygenation on hepatocytes, and can be reversed by defatting.
Figures
References
-
- Busuttil RW, Tanaka K. The utility of marginal donors in liver transplantation. Liver Transpl. 2003;9:651–663. - PubMed
-
- Adam R, Reynes M, Johann M, Morino M, Astarcioglu I, Kafetzis I, Castaing D, Bismuth H. The outcome of steatotic grafts in liver transplantation. Transplant Proc. 1991;23:1538–1540. - PubMed
-
- Chui AK, Shi LW, Rao AR, Verran DJ, Painter D, Koorey D, McCaughan GW, Sheil AG. Donor fatty (steatotic) liver allografts in orthotopic liver transplantation: a revisit. Transplant Proc. 2000;32:2101–2102. - PubMed
-
- Nakamuta M, Morizono S, Soejima Y, Yoshizumi T, Aishima S, Takasugi S, Yoshimitsu K, Enjoji M, Kotoh K, Taketomi A, Uchiyama H, Shimada M, Nawata H, Maehara Y. Short-term intensive treatment for donors with hepatic steatosis in living-donor liver transplantation. Transplantation. 2005;80:608–612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

