Functional properties and differential neuromodulation of Na(v)1.6 channels
- PMID: 18599309
- PMCID: PMC3433175
- DOI: 10.1016/j.mcn.2008.05.009
Functional properties and differential neuromodulation of Na(v)1.6 channels
Abstract
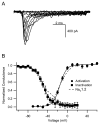
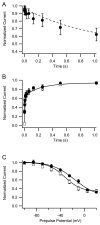
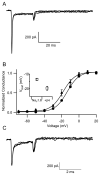
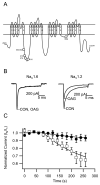
The voltage-gated sodium channel Na(v)1.6 plays unique roles in the nervous system, but its functional properties and neuromodulation are not as well established as for Na(V)1.2 channels. We found no significant differences in voltage-dependent activation or fast inactivation between Na(V)1.6 and Na(V)1.2 channels expressed in non-excitable cells. In contrast, the voltage dependence of slow inactivation was more positive for Na(v)1.6 channels, they conducted substantially larger persistent sodium currents than Na(v)1.2 channels, and they were much less sensitive to inhibition by phosphorylation by cAMP-dependent protein kinase and protein kinase C. Resurgent sodium current, a hallmark of Na(v)1.6 channels in neurons, was not observed for Na(V)1.6 expressed alone or with the auxiliary beta(4) subunit. The unique properties of Na(V)1.6 channels, together with the resurgent currents that they conduct in neurons, make these channels well-suited to provide the driving force for sustained repetitive firing, a crucial property of neurons.
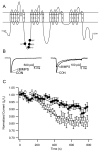
Figures

References
-
- Balser JR. Inherited sodium channelopathies: novel therapeutic and proarrhythmic molecular mechanisms. Trends Cardiovasc Med. 2002;11:229–237. - PubMed
-
- Boiko T, Rasband MN, Levinson SR, Caldwell JH, Mandel G, Trimmer JS, Matthews G. Compact myelin dictates the differential targeting of two sodium channel isoforms in the same axon. Neuron. 2001;30:91–104. - PubMed
-
- Burbidge SA, Dale TJ, Powell AJ, Whitaker WR, Xie XM, Romanos MA, Clare JJ. Molecular cloning, distribution and functional analysis of the NA(V)1.6. Voltage-gated sodium channel from human brain. Mol Brain Res. 2002;103:80–90. - PubMed
-
- Burgess DL, Kohrman DC, Galt J, Plummer NW, Jones JM, Spear B, Meisler MH. Mutation of a new sodium channel gene, Scn8a, in the mouse mutant ‘motor endplate disease’. Nat Genet. 1995;10:461–465. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

