MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function
- PMID: 18599438
- PMCID: PMC2453723
- DOI: 10.1073/pnas.0802679105
MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function
Abstract
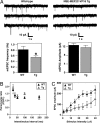
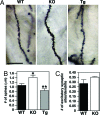
Learning and memory depend on the activity-dependent structural plasticity of synapses and changes in neuronal gene expression. We show that deletion of the MEF2C transcription factor in the CNS of mice impairs hippocampal-dependent learning and memory. Unexpectedly, these behavioral changes were accompanied by a marked increase in the number of excitatory synapses and potentiation of basal and evoked synaptic transmission. Conversely, neuronal expression of a superactivating form of MEF2C results in a reduction of excitatory postsynaptic sites without affecting learning and memory performance. We conclude that MEF2C limits excessive synapse formation during activity-dependent refinement of synaptic connectivity and thus facilitates hippocampal-dependent learning and memory.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Chen A, et al. Inducible enhancement of memory storage and synaptic plasticity in transgenic mice expressing an inhibitor of atf4 (creb-2) and c/ebp proteins. Neuron. 2003;39:655–669. - PubMed
-
- Potthoff MJ, Olson EN. Mef2: A central regulator of diverse developmental programs. Development. 2007;134:4131–4140. - PubMed
-
- Leifer D, Golden J, Kowall NW. Myocyte-specific enhancer binding factor 2c expression in human brain development. Neuroscience. 1994;63:1067–1079. - PubMed
-
- Flavell SW, et al. Activity-dependent regulation of mef2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

