Dependence of antibody-mediated presentation of antigen on FcRn
- PMID: 18599440
- PMCID: PMC2453734
- DOI: 10.1073/pnas.0801717105
Dependence of antibody-mediated presentation of antigen on FcRn
Abstract
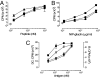
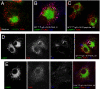
The neonatal Fc receptor for IgG (FcRn) is a distant member of the MHC class I protein family. It binds IgG and albumin in a pH-dependent manner and protects these from catabolism by diverting them from a degradative fate in lysosomes. In addition, FcRn-mediated IgG transport across epithelial barriers is responsible for the transmission of IgG from mother to infant and can also enhance IgG-mediated antigen uptake across mucosal epithelia. We now show a previously undescribed role for FcRn in mediating the presentation of antigens by dendritic cells when antigens are present as a complex with antibody by uniquely directing multimeric immune complexes, but not monomeric IgG, to lysosomes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

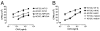



References
-
- Liu Y, et al. Regulated expression of FcγR in human dendritic cells controls cross-presentation of antigen-antibody complexes. J Immunol. 2006;177:8440–8447. - PubMed
-
- Ravetch JV, Bolland S. IgG Fc receptors. Annu Rev Immunol. 2001;19:275–290. - PubMed
-
- Hirano M, et al. IgEb immune complexes activate macrophages through FcγRIV binding. Nat Immunol. 2007;8:762–771. - PubMed
-
- Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337:184–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

