Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs
- PMID: 18599452
- PMCID: PMC2474514
- DOI: 10.1073/pnas.0801866105
Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs
Abstract
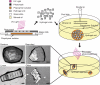
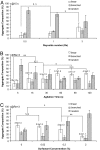
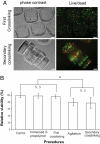
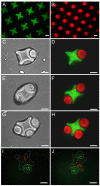
We present a bottom-up approach to direct the assembly of cell-laden microgels to generate tissue constructs with tunable microarchitecture and complexity. This assembly process is driven by the tendency of multiphase liquid-liquid systems to minimize the surface area and the resulting surface free energy between the phases. We demonstrate that shape-controlled microgels spontaneously assemble within multiphase reactor systems into predetermined geometric configurations. Furthermore, we characterize the parameters that influence the assembly process, such as external energy input, surface tension, and microgel dimensions. Finally, we show that multicomponent cell-laden constructs could be generated by assembling microgel building blocks and performing a secondary cross-linking reaction. This bottom-up approach for the directed assembly of cell-laden microgels provides a powerful and highly scalable approach to form biomimetic 3D tissue constructs and opens a paradigm for directing the assembly of mesoscale materials.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Costanzo L. Physiology. 3rd Ed. Philadelphia: Saunders; 2006.
-
- Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–926. - PubMed
-
- Griffith LG, Swartz MA. Capturing complex 3D tissue physiology in vitro. Nat Rev Mol Cell Biol. 2006;7:211–224. - PubMed
-
- Levenberg S, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–884. - PubMed
-
- Khademhosseini A, Langer R. Microengineered hydrogels for tissue engineering. Biomaterials. 2007;28:5087–5092. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

