Evolution of the phospho-tyrosine signaling machinery in premetazoan lineages
- PMID: 18599463
- PMCID: PMC2443182
- DOI: 10.1073/pnas.0803161105
Evolution of the phospho-tyrosine signaling machinery in premetazoan lineages
Abstract
Multicellular animals use a three-part molecular toolkit to mediate phospho-tyrosine signaling: Tyrosine kinases (TyrK), protein tyrosine phosphatases (PTP), and Src Homology 2 (SH2) domains function, respectively, as "writers," "erasers," and "readers" of phospho-tyrosine modifications. How did this system of three components evolve, given their interdependent function? Here, we examine the usage of these components in 41 eukaryotic genomes, including the newly sequenced genome of the choanoflagellate, Monosiga brevicollis, the closest known unicellular relative to metazoans. This analysis indicates that SH2 and PTP domains likely evolved earliest-a handful of these domains are found in premetazoan eukaryotes lacking tyrosine kinases, most likely to deal with limited tyrosine phosphorylation cross-catalyzed by promiscuous Ser/Thr kinases. Modern TyrK proteins, however, are only observed in two lineages, metazoans and choanoflagellates. These two lineages show a dramatic coexpansion of all three domain families. Concurrent expansion of the three domain families is consistent with a stepwise evolutionary model in which preexisting SH2 and PTP domains were of limited utility until the appearance of the TyrK domain in the last common ancestor of metazoans and choanoflagellates. The emergence of the full three-component signaling system, with its dramatically increased encoding potential, may have contributed to the advent of metazoan multicellularity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
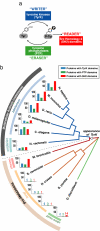
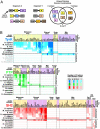


Comment in
-
Clues to the evolution of complex signaling machinery.Proc Natl Acad Sci U S A. 2008 Jul 15;105(28):9453-4. doi: 10.1073/pnas.0804669105. Epub 2008 Jul 10. Proc Natl Acad Sci U S A. 2008. PMID: 18621723 Free PMC article. Review. No abstract available.
References
-
- Myers MG, et al. Insulin receptor substrate-1 mediates phosphatidylinositol 3′-kinase and p70S6k signaling during insulin, insulin-like growth factor-1, and interleukin-4 stimulation. J Biol Chem. 1994;269:28783–28789. - PubMed
-
- Hunter T, Cooper JA. Epidermal growth factor induces rapid tyrosine phosphorylation of proteins in A431 human tumor cells. Cell. 1981;24:741–752. - PubMed
-
- Weiss A, Littman DR. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. - PubMed
-
- Shattil SJ, Brugge JS. Protein tyrosine phosphorylation and the adhesive functions of platelets. Curr Opin Cell Biol. 1991;3:869–879. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

