Supramolecular interactions in the dermo-epidermal junction zone: anchoring fibril-collagen VII tightly binds to banded collagen fibrils
- PMID: 18599485
- PMCID: PMC3259843
- DOI: 10.1074/jbc.M802415200
Supramolecular interactions in the dermo-epidermal junction zone: anchoring fibril-collagen VII tightly binds to banded collagen fibrils
Abstract
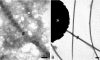
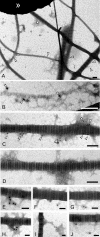
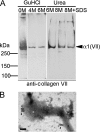
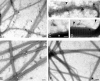
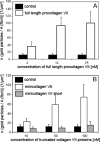
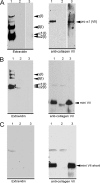
The dermis and the epidermis of normal human skin are functionally separated by a basement membrane but, together, form a stable structural continuum. Anchoring fibrils reinforce this connection by insertion into the basement membrane and by intercalation with banded collagen fibrils of the papillary dermis. Structural abnormalities in collagen VII, the major molecular constituent of anchoring fibrils, lead to a congenital skin fragility condition, dystrophic epidermolysis bullosa, associated with skin blistering. Here, we characterized the molecular basis of the interactions between anchoring fibrils and banded collagen fibrils. Suprastructural fragments of the dermo-epidermal junction zone were generated by mechanical disruption and by separation with magnetic Immunobeads. Anchoring fibrils were tightly attached to banded collagen fibrils. In vitro binding studies demonstrated that a von Willebrand factor A-like motif in collagen VII was essential for binding of anchoring fibrils to reconstituted collagen I fibrils. Since collagen I and VII molecules reportedly undergo only weak interactions, the attachment of anchoring fibrils to collagen fibrils depends on supramolecular organization of their constituents. This complex is stabilized in situ and resists dissociation by strong denaturants.
Figures



References
-
- Ellison, J., and Garrod, D. R. (1984) J. Cell Sci. 72 163–172 - PubMed
-
- Burgeson, R. E., Lunstrum, G. P., Rokosova, B., Rimberg, C. S., Rosenbaum, L. M., and Keene, D. R. (1990) Ann. N. Y. Acad. Sci. 580 32–43 - PubMed
-
- Shimizu, H., Ishiko, A., Masunaga, T., Kurihara, Y., Sato, M., Bruckner-Tuderman, L., and Nishikawa, T. (1997) Lab. Investig. 76 753–763 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

