Vesicular apparatus, including functional calcium channels, are present in developing rodent optic nerve axons and are required for normal node of Ranvier formation
- PMID: 18599536
- PMCID: PMC2652192
- DOI: 10.1113/jphysiol.2008.155077
Vesicular apparatus, including functional calcium channels, are present in developing rodent optic nerve axons and are required for normal node of Ranvier formation
Abstract
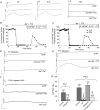
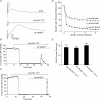
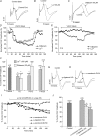
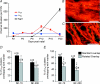
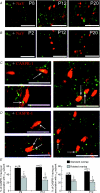
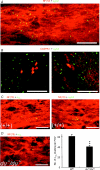
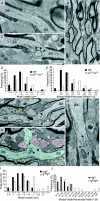
P/Q-type calcium channels are known to form clusters at the presynaptic membrane where they mediate calcium influx, triggering vesicle fusion. We now report functional P/Q channel clusters in the axolemma of developing central axons that are also associated with sites of vesicle fusion. These channels were activated by axonal action potentials and the resulting calcium influx is well suited to mediate formation of a synaptic style SNARE complex involving SNAP-25, that we show to be located on the axolemma. Vesicular elements within axons were found to be the sole repository of vesicular glutamate in developing white matter. The axonal vesicular elements expressed the glutamate transporter V-ATPase, which is responsible for vesicular glutamate loading. The P/Q channel alpha(1A) subunit was found to be present within the axolemma at early nodes of Ranvier and deleterious mutations of the alpha(1A) subunit, or an associated alpha(2)delta-2 subunit, disrupted the localization of nodal proteins such as voltage-gated sodium channels, beta IV spectrin and CASPR-1. This was associated with the presence of malformed nodes of Ranvier characterized by an accumulation of axoplasmic vesicles under the nodal membrane. The data are consistent with the presence of a vesicular signalling pathway between axons and glial cells that is essential for proper development of the node of Ranvier.
Figures



References
-
- Aidley DJ. The Physiology of Excitable Cells. Cambridge: Cambridge University Press; 1971.
-
- Back SA, Craig A, Kayton RJ, Luo NL, Meshul CK, Allcock N, Fern R. Hypoxia-ischemia preferentially triggers glutamate depletion from oligodendroglia and axons in perinatal cerebral white matter. J Cereb Blood Flow Metab. 2006;27:334–347. - PubMed
-
- Back SA, Rivkees SA. Emerging concepts in periventricular white matter injury. Semin Perinatol. 2004;28:405–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

