Tomato protein kinase 1b mediates signaling of plant responses to necrotrophic fungi and insect herbivory
- PMID: 18599583
- PMCID: PMC2518242
- DOI: 10.1105/tpc.108.059477
Tomato protein kinase 1b mediates signaling of plant responses to necrotrophic fungi and insect herbivory
Abstract
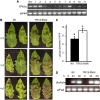
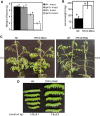
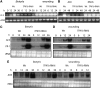
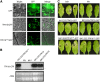
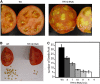
The tomato protein kinase 1 (TPK1b) gene encodes a receptor-like cytoplasmic kinase localized to the plasma membrane. Pathogen infection, mechanical wounding, and oxidative stress induce expression of TPK1b, and reducing TPK1b gene expression through RNA interference (RNAi) increases tomato susceptibility to the necrotrophic fungus Botrytis cinerea and to feeding by larvae of tobacco hornworm (Manduca sexta) but not to the bacterial pathogen Pseudomonas syringae. TPK1b RNAi seedlings are also impaired in ethylene (ET) responses. Notably, susceptibility to Botrytis and insect feeding is correlated with reduced expression of the proteinase inhibitor II gene in response to Botrytis and 1-aminocyclopropane-1-carboxylic acid, the natural precursor of ET, but wild-type expression in response to mechanical wounding and methyl-jasmonate. TPK1b functions independent of JA biosynthesis and response genes required for resistance to Botrytis. TPK1b is a functional kinase with autophosphorylation and Myelin Basis Protein phosphorylation activities. Three residues in the activation segment play a critical role in the kinase activity and in vivo signaling function of TPK1b. In sum, our findings establish a signaling role for TPK1b in an ET-mediated shared defense mechanism for resistance to necrotrophic fungi and herbivorous insects.
Figures
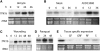





References
-
- AbuQamar, S., Chen, X., Dahwan, R., Bluhm, B., Salmeron, J., Lam, S., Dietrich, R.A., and Mengiste, T. (2006). Expression profiling and mutant analysis reveals complex regulatory networks involved in Arabidopsis response to Botrytis infection. Plant J. 48 28–44. - PubMed
-
- Asai, T., Tena, G., Plotnikova, J., Willmann, M.R., Chiu, W.L., Gomez-Gomez, L., Boller, T., Ausubel, F.M., and Sheen, J. (2002). MAP kinase signaling cascade in Arabidopsis innate immunity. Nature 415 977–983. - PubMed
-
- Benito, E.P., ten Have, A., Van Klooster, J.W., and van Kan, J.A.L. (1998). Fungal and plant gene expression during synchronized infection of tomato leaves by Botrytis cinerea. Eur. J. Plant Pathol. 104 207–220.
-
- Berrocal-Lobo, M., Molina, A., and Solano, R. (2002). Constitutive expression of ETHYLENE-RESPONSE-FACTOR 1 in Arabidopsis confers resistance to several necrotrophic fungi. Plant J. 29 23–32. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

