Protein kinase A-mediated phosphorylation of cMyBP-C increases proximity of myosin heads to actin in resting myocardium
- PMID: 18599866
- PMCID: PMC2810832
- DOI: 10.1161/CIRCRESAHA.108.178996
Protein kinase A-mediated phosphorylation of cMyBP-C increases proximity of myosin heads to actin in resting myocardium
Abstract
Protein kinase A-mediated (PKA) phosphorylation of cardiac myosin binding protein C (cMyBP-C) accelerates the kinetics of cross-bridge cycling and may relieve the tether-like constraint of myosin heads imposed by cMyBP-C. We favor a mechanism in which cMyBP-C modulates cross-bridge cycling kinetics by regulating the proximity and interaction of myosin and actin. To test this idea, we used synchrotron low-angle x-ray diffraction to measure interthick filament lattice spacing and the equatorial intensity ratio, I(11)/I(10), in skinned trabeculae isolated from wild-type and cMyBP-C null (cMyBP-C(-/-)) mice. In wild-type myocardium, PKA treatment appeared to result in radial or azimuthal displacement of cross-bridges away from the thick filaments as indicated by an increase (approximately 50%) in I(11)/I(10) (0.22+/-0.03 versus 0.33+/-0.03). Conversely, PKA treatment did not affect cross-bridge disposition in mice lacking cMyBP-C, because there was no difference in I(11)/I(10) between untreated and PKA-treated cMyBP-C(-/-) myocardium (0.40+/-0.06 versus 0.42+/-0.05). Although lattice spacing did not change after treatment in wild-type (45.68+/-0.84 nm versus 45.64+/-0.64 nm), treatment of cMyBP-C(-/-) myocardium increased lattice spacing (46.80+/-0.92 nm versus 49.61+/-0.59 nm). This result is consistent with the idea that the myofilament lattice expands after PKA phosphorylation of cardiac troponin I, and when present, cMyBP-C, may stabilize the lattice. These data support our hypothesis that tethering of cross-bridges by cMyBP-C is relieved by phosphorylation of PKA sites in cMyBP-C, thereby increasing the proximity of cross-bridges to actin and increasing the probability of interaction with actin on contraction.
Figures

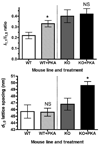


References
-
- de Tombe PP. Myosin binding protein C in the heart. Circ Res. 2006;98:1234–1236. - PubMed
-
- Winegrad S. Myosin binding protein-C – a potential regulator of cardiac contractility. Circ Res. 2002;86:6–7. - PubMed
-
- Stelzer JE, Patel JR, Moss RL. Protein kinase-A mediated acceleration of the stretch activation response in murine skinned myocardium is eliminated by ablation of cMyBP-C. Circ Res. 2006;99:884–890. - PubMed
-
- Stelzer JE, Patel JR, Walker JW, Moss RL. Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation. Circ Res. 2007;101:503–511. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

