E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas
- PMID: 18600305
- PMCID: PMC2929356
- DOI: 10.1007/s10585-008-9167-1
E-cadherin as an indicator of mesenchymal to epithelial reverting transitions during the metastatic seeding of disseminated carcinomas
Abstract
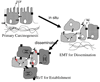
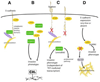
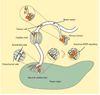
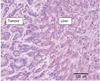
Cancer metastasis follows a sequential series of events, and many of the critical steps are distinctly similar to EMT-like transformations that occur during normal embryonic development. A current area of focus is the similarities between how cancer cells interact with the ectopic parenchyma after metastatic spread, and secondary developmental MET events that occur in epithelial tissues that have re-assembled within the embryo from mesenchymal cells. Accumulating evidence suggests a critical role for these secondary events, termed mesenchymal-epithelial transitions (MET) in development and mesenchymal-epithelial reverting transitions (MErT) in cancer. In this situation, metastatic seed cancer cells may inertly become part of the ectopic tissue and therefore surmount the metastatic inefficiencies to which most disseminated cancer cells succumb. Just as a critical EMT event is the downregulation or silencing of E-cadherin, we discuss the role of E-cadherin in cancer-associated MErT at distant metastatic sites and speculate on the implications for the fate of micrometastases that undergo a transition to being E-cadherin positive.
Figures

References
-
- Babu M, Wells A. Dermal-epidermal communication in wound healing. Wounds. 2001;13:183–189.
-
- Birchmeier C, Birchmeier W, Gherardi E, VandeWoude GF. Met, metastasis, motility and more. Nat Rev Mol Cell Biol. 2003;4:915–925. - PubMed
-
- Boccaccio C, Comoglio PM. Invasive growth: a MET-driven genetic programme for cancer and stem cells. Nat Rev Cancer. 2006;6:637–645. - PubMed
-
- Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319–8326. - PubMed
-
- Christofori G. New signals from the invasive front. Nature. 2006;441:444–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

