The IFN-gamma-induced transcriptional program of the CIITA gene is inhibited by statins
- PMID: 18601229
- PMCID: PMC2692880
- DOI: 10.1002/eji.200838189
The IFN-gamma-induced transcriptional program of the CIITA gene is inhibited by statins
Abstract
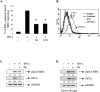
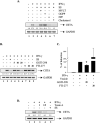
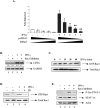
Statins are 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors that exert anti-inflammatory effects. IFN-gamma induction of class II MHC expression, which requires the class II transactivator (CIITA), is inhibited by statins; however, the molecular basis for suppression is undetermined. We describe that statins inhibit IFN-gamma-induced class II MHC expression by suppressing CIITA gene expression, which is dependent on the HMG-CoA reductase pathway. In addition, CIITA expression is inhibited by GGTI-298 or Clostridium difficile Toxin A, specific inhibitors of Rho family protein prenylation, indicating the involvement of small GTPases. Rac1 is involved in IFN-gamma inducible expression of CIITA, and statins inhibit IFN-gamma-induced Rac1 activation, contributing to the inhibitory effect of statins. IFN-gamma induction of the CIITA gene is regulated by the transcription factors STAT-1alpha, interferon regulatory factor (IRF)-1 and upstream stimulatory factor (USF)-1. We previously reported that statins inhibit constitutive STAT-1alpha expression. IRF-1, a STAT-1 dependent gene, is also inhibited by statins. Therefore, statin treatment results in decreased recruitment of STAT-1alpha and IRF-1 to the endogenous CIITA promoter IV (pIV). The recruitment of USF-1 to CIITA pIV is also reduced by statins, as is the recruitment of RNA polymerase II (Pol II), p300 and Brg-1. These data indicate that statins inhibit the transcriptional program of the CIITA gene.
Figures

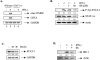

Similar articles
-
Inhibition of interferon-gamma-mediated microvascular endothelial cell major histocompatibility complex class II gene activation by HMG-CoA reductase inhibitors.Transplantation. 2001 May 15;71(9):1262-8. doi: 10.1097/00007890-200105150-00014. Transplantation. 2001. PMID: 11397960
-
IFN-gamma regulation of class II transactivator promoter IV in macrophages and microglia: involvement of the suppressors of cytokine signaling-1 protein.J Immunol. 2001 Feb 15;166(4):2260-9. doi: 10.4049/jimmunol.166.4.2260. J Immunol. 2001. PMID: 11160280
-
IFN-gamma regulation of the type IV class II transactivator promoter in astrocytes.J Immunol. 1999 Apr 15;162(8):4731-9. J Immunol. 1999. PMID: 10202014
-
The HMG-CoA reductase inhibitor simvastatin inhibits IFN-gamma induced MHC class II expression in human vascular endothelial cells.Swiss Med Wkly. 2001 Jan 27;131(3-4):41-6. doi: 10.4414/smw.2001.06144. Swiss Med Wkly. 2001. PMID: 11219190
-
A novel pleiotropic effect of statins: prevention of cardiac hypertrophy by cholesterol-independent mechanisms.Ann Med. 2003;35(6):398-403. doi: 10.1080/07853890310001294. Ann Med. 2003. PMID: 14572163 Free PMC article. Review.
Cited by
-
Potential biological functions of cytochrome P450 reductase-dependent enzymes in small intestine: novel link to expression of major histocompatibility complex class II genes.J Biol Chem. 2012 May 18;287(21):17777-17788. doi: 10.1074/jbc.M112.354274. Epub 2012 Mar 27. J Biol Chem. 2012. PMID: 22453923 Free PMC article.
-
Coronavirus infection: An immunologists' perspective.Scand J Immunol. 2021 Jun;93(6):e13043. doi: 10.1111/sji.13043. Epub 2021 Apr 7. Scand J Immunol. 2021. PMID: 33783027 Free PMC article. Review.
-
Beyond pattern recognition: NOD-like receptors in dendritic cells.Trends Immunol. 2013 May;34(5):224-33. doi: 10.1016/j.it.2012.12.003. Epub 2013 Jan 23. Trends Immunol. 2013. PMID: 23352728 Free PMC article. Review.
-
Anti-Inflammatory Effects of Lipid-Lowering Drugs and Supplements-A Narrative Review.Nutrients. 2023 Mar 21;15(6):1517. doi: 10.3390/nu15061517. Nutrients. 2023. PMID: 36986246 Free PMC article. Review.
-
Stable protein, unstable plaque?J Mol Cell Cardiol. 2009 Mar;46(3):289-91. doi: 10.1016/j.yjmcc.2008.11.013. Epub 2008 Dec 3. J Mol Cell Cardiol. 2009. PMID: 19101562 Free PMC article. No abstract available.
References
-
- Turesson C. Endothelial expression of MHC class II molecules in autoimmune disease. Curr. Pharm. Des. 2004;10:129–143. - PubMed
-
- O'Keefe GM, Nguyen VT, Benveniste EN. Regulation and function of class II major histocompatibility complex, CD40, and B7 expression in macrophages and microglia: Implications in neurological diseases. J. Neurovirol. 2002;8:496–512. - PubMed
-
- Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85:307–310. - PubMed
-
- Dyment DA, Sadovnick AD, Ebers GC. Genetics of multiple sclerosis. Hum. Mol. Genet. 1997;6:1693–1698. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

