Aggregation and catabolism of disease-associated intra-Abeta mutations: reduced proteolysis of AbetaA21G by neprilysin
- PMID: 18602473
- PMCID: PMC3160758
- DOI: 10.1016/j.nbd.2008.06.001
Aggregation and catabolism of disease-associated intra-Abeta mutations: reduced proteolysis of AbetaA21G by neprilysin
Abstract
Five point mutations within the amyloid beta-protein (Abeta) sequence of the APP gene are associated with hereditary diseases which are similar or identical to Alzheimer's disease and encode: the A21G (Flemish), E22G (Arctic), E22K (Italian), E22Q (Dutch) and the D23N (Iowa) amino acid substitutions. Although a substantial body of data exists on the effects of these mutations on Abeta production, whether or not intra-Abeta mutations alter degradation and how this relates to their aggregation state remain unclear. Here we report that the E22G, E22Q and the D23N substitutions significantly increase fibril nucleation and extension, whereas the E22K substitution exhibits only an increased rate of extension and the A21G substitution actually causes a decrease in the extension rate. These substantial differences in aggregation together with our observation that aggregated wild type Abeta(1-40) was much less well degraded than monomeric wild type Abeta(1-40), prompted us to assess whether or not disease-associated intra-Abeta mutations alter proteolysis independent of their effects on aggregation. Neprilysin (NEP), insulin degrading enzyme (IDE) and plasmin play a major role in Abeta catabolism, therefore we compared the ability of these enzymes to degrade wild type and mutant monomeric Abeta peptides. Experiments investigating proteolysis revealed that all monomeric peptides are degraded similarly by IDE and plasmin, but that the Flemish peptide was degraded significantly more slowly by NEP than wild type Abeta or any of the other mutant peptides. This finding suggests that resistance to NEP-mediated proteolysis may underlie the pathogenicity associated with the A21G mutation.
Figures

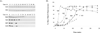
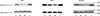


References
-
- Beavis RC, Chait BT. Matrix-assisted laser desorption ionization mass-spectrometry of proteins. Methods Enzymol. 1996;270:519–551. - PubMed
-
- Brooks WS, Kwok JB, Halliday GM, Godbolt AK, Rossor MN, Creasey H, Jones AO, Schofield PR. Hemorrhage is uncommon in new Alzheimer family with Flemish amyloid precursor protein mutation. Neurology. 2004;63:1613–1617. - PubMed
-
- Cacquevel M, Launay S, Castel H, Benchenane K, Cheenne S, Buee L, Moons L, Delacourte A, Carmeliet P, Vivien D. Ageing and amyloid-beta peptide deposition contribute to an impaired brain tissue plasminogen activator activity by different mechanisms. Neurobiol Dis. 2007;27:164–173. - PubMed
-
- Carson JA, Turner AJ. beta-amyloid catabolism: roles for neprilysin (NEP) and other metallopeptidases? Journal of Neurochemistry. 2002;81:1–8. - PubMed
-
- Cheng IH, Palop JJ, Esposito LA, Bien-Ly N, Yan F, Mucke L. Aggressive amyloidosis in mice expressing human amyloid peptides with the Arctic mutation. Nat Med. 2004;10:1190–1192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

