PEDF-deficient mice exhibit an enhanced rate of retinal vascular expansion and are more sensitive to hyperoxia-mediated vessel obliteration
- PMID: 18602915
- PMCID: PMC2562453
- DOI: 10.1016/j.exer.2008.06.003
PEDF-deficient mice exhibit an enhanced rate of retinal vascular expansion and are more sensitive to hyperoxia-mediated vessel obliteration
Abstract
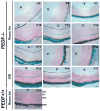
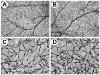
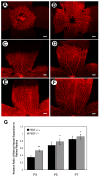
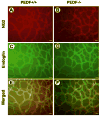
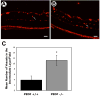
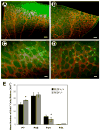
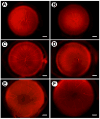
Pigment epithelium derived factor (PEDF) is an endogenous inhibitor of angiogenesis. However, its physiological role during vascular development and neovascularization remains elusive. Here we investigated the role of PEDF in normal postnatal vascularization of retina and retinal neovascularization during oxygen-induced ischemic retinopathy (OIR) using PEDF-deficient (PEDF-/-) mice. The beta-galactosidase staining of eye sections from PEDF-/- mice confirmed the expression pattern of endogenous PEDF previously reported in mouse retina. However, strongest staining was observed in the retinal outer plexiform layer. Retinal trypsin digests indicated that the ratio of endothelial cells (EC) to pericytes (PC) was significantly higher in PEDF-/- mice compared to wild type (PEDF+/+) mice at postnatal day 21 (P21). This was mainly attributed to increased numbers of EC in the absence of PEDF. There was no significant difference in the number of PC. We observed an increased rate of proliferation in retinal vasculature of PEDF-/- mice, which was somewhat compensated for by an increase in the rate of apoptosis. Staining of the retinal wholemounts and eye frozen sections indicated postnatal retinal vascularization expansion occurred at a faster rate in the absence of PEDF, and was more prominent at early time points (prior to P21). The retinal vascularization in PEDF+/+ mice reaches that of PEDF-/- mice such that no significant difference in vascular densities was observed by P42. Lack of PEDF had a minimal effect on the regression of hyaloid vasculature and VEGF levels. PEDF-/- mice also exhibited enhanced sensitivity to hyperoxia-mediated vessel obliteration during OIR compared to PEDF+/+ mice despite higher levels of VEGF. However, there was no significant difference in the degree of retinal neovascularization. Our studies indicate that PEDF is an important modulator of early postnatal retinal vascularization and in its absence retinal vascularization proceeds at a faster rate and is more susceptible to hyperoxia-mediated vessel obliteration.
Figures






References
-
- Amano S, Yamagishi S, Inagaki Y, Nakamura K, Takeuchi M, Inoue H, Imaizumi T. Pigment epithelium-derived factor inhibits oxidative stress-induced apoptosis and dysfunction of cultured retinal pericytes. Microvasc Res. 2005;69(1–2):45–55. - PubMed
-
- Becerra SP. Structure-function studies on PEDF. A noninhibitory serpin with neurotrophic activity. Adv Exp Med Biol. 1997;425:223–237. - PubMed
-
- Becerra SP, Sagasti A, Spinella P, Notario V. Pigment epithelium-derived factor behaves like a noninhibitory serpin. Neurotrophic activity does not require the serpin reactive loop. J Biol Chem. 1995;270(43):25992–25999. - PubMed
-
- Behling KC, Surace EM, Bennett J. Pigment epithelium-derived factor expression in the developing mouse eye. Mol Vis. 2002;8:449–454. - PubMed
-
- Bhutto IA, Kim SY, McLeod DS, Merges C, Fukai N, Olsen BR, Lutty GA. Localization of collagen XVIII and the endostatin portion of collagen XVIII in aged human control eyes and eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2004;45(5):1544–1552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

