Reduced pulsatility induces periarteritis in kidney: role of the local renin-angiotensin system
- PMID: 18603068
- PMCID: PMC2533270
- DOI: 10.1016/j.jtcvs.2007.12.023
Reduced pulsatility induces periarteritis in kidney: role of the local renin-angiotensin system
Abstract
Objective: The need for pulsatility in the circulation during long-term mechanical support has been a subject of debate. We compared histologic changes in calf renal arteries subjected to various degrees of pulsatile circulation in vivo. We addressed the hypothesis that the local renin-angiotensin system may be implicated in these histologic changes.
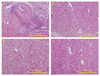
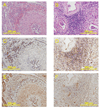
Methods and results: Sixteen calves were implanted with devices giving differing degrees of pulsatile circulation: 6 had a continuous flow left ventricular assist device (LVAD); 6 had a continuous flow right ventricular assist device (RVAD); and 4 had a pulsatile total artificial heart (TAH). Six other calves were histologic and immunohistochemical controls. In the LVAD group, the pulsatility index was significantly lower (0.28 +/- 0.07 LVAD vs 0.56 +/- 0.08 RVAD, vs 0.53 +/- 0.10 TAH; P < 0.01), and we observed severe periarteritis in all cases in the LVAD group. The number of angiotensin II type 1 receptor-positive cells and angiotensin converting enzyme-positive cells in periarterial areas was significantly higher in the LVAD group (angiotensin II type 1 receptor: 350 +/- 139 LVAD vs 8 +/- 6 RVAD, vs 3 +/- 2 TAH, vs 3 +/- 2 control; P < .001; angiotensin-converting enzyme: 325 +/- 59 LVAD vs 6 +/- 4 RVAD, vs 6 +/- 5 TAH, vs 3 +/- 1 control; P < .001).
Conclusions: The reduced pulsatility produced by a continuous flow LVAD implantation induced severe periarteritis in the kidneys. The local renin-angiotensin system was up-regulated in the inflammatory cells only in the continuous flow LVAD group.
Figures





References
-
- Nishimura T, Tatsumi E, Takaichi S, Taenaka Y, Wakisaka Y, Nakatani T, Masuzawa T, Takewa Y, Nakamura M, Endo S, Nakata M, Takano H. Prolonged nonpulsatile left heart bypass with reduced systemic pulse pressure causes morphological changes in the aortic wall. Artif Org. 1998;22:405–410. - PubMed
-
- Kihara S, Litwak KN, Nichols L, Litwak P, Kameneva MV, Wu Z, Kormos RL, Griffith BP. Smooth muscle cell hypertrophy of renal cortex arteries with chronic continuous flow left ventricular assist. Ann Thorac Surg. 2003;75:178–183. - PubMed
-
- Leung PS. The peptide hormone angiotensin II: its new functions in tissues and organs. Curr Protein Pept Sci. 2004;5:267–273. - PubMed
-
- Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacol Rev. 2000;52:11–34. - PubMed
-
- Dostal DE, Baker KM. The cardiac renin-angiotensin system: conceptual, or a regulator of cardiac function? Circ Res. 1999;85:643–650. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

