Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes
- PMID: 18603259
- PMCID: PMC2654363
- DOI: 10.1016/j.yjmcc.2008.05.014
Emerging roles of inositol 1,4,5-trisphosphate signaling in cardiac myocytes
Abstract
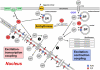
Inositol 1,4,5-trisphosphate (IP(3)) is a ubiquitous intracellular messenger regulating diverse functions in almost all mammalian cell types. It is generated by membrane receptors that couple to phospholipase C (PLC), an enzyme which liberates IP(3) from phosphatidylinositol 4,5-bisphosphate (PIP(2)). The major action of IP(3), which is hydrophilic and thus translocates from the membrane into the cytoplasm, is to induce Ca(2+) release from endogenous stores through IP(3) receptors (IP(3)Rs). Cardiac excitation-contraction coupling relies largely on ryanodine receptor (RyR)-induced Ca(2+) release from the sarcoplasmic reticulum. Myocytes express a significantly larger number of RyRs compared to IP(3)Rs (~100:1), and furthermore they experience substantial fluxes of Ca(2+) with each heartbeat. Therefore, the role of IP(3) and IP(3)-mediated Ca(2+) signaling in cardiac myocytes has long been enigmatic. Recent evidence, however, indicates that despite their paucity cardiac IP(3)Rs may play crucial roles in regulating diverse cardiac functions. Strategic localization of IP(3)Rs in cytoplasmic compartments and the nucleus enables them to participate in subsarcolemmal, bulk cytoplasmic and nuclear Ca(2+) signaling in embryonic stem cell-derived and neonatal cardiomyocytes, and in adult cardiac myocytes from the atria and ventricles. Intriguingly, expression of both IP(3)Rs and membrane receptors that couple to PLC/IP(3) signaling is altered in cardiac disease such as atrial fibrillation or heart failure, suggesting the involvement of IP(3) signaling in the pathology of these diseases. Thus, IP(3) exerts important physiological and pathological functions in the heart, ranging from the regulation of pacemaking, excitation-contraction and excitation-transcription coupling to the initiation and/or progression of arrhythmias, hypertrophy and heart failure.
Figures




References
-
- Streb H, Irvine RF, Berridge MJ, Schulz I. Release of Ca2+ from a nonmitochondrial intracellular store in pancreatic acinar cells by inositol-1,4,5-trisphosphate. Nature. 1983;306:67–9. - PubMed
-
- Rizzuto R, Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86:369–408. - PubMed
-
- Woodcock EA, Lambert KA, Phan T, Jacobsen AN. Inositol phosphate metabolism during myocardial ischemia. J Mol Cell Cardiol. 1997;29:449–60. - PubMed
-
- Nasuhoglu C, Feng S, Mao Y, Shammat I, Yamamato M, Earnest S, et al. Modulation of cardiac PIP2 by cardioactive hormones and other physiologically relevant interventions. Am J Physiol Cell Physiol. 2002;283:C223–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL080101/HL/NHLBI NIH HHS/United States
- BBS/E/B/00001116/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/B/0000H182/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/B/0000H215/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/B/0000H157/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/B/0000C116/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/B/0000C134/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- R01 HL062231/HL/NHLBI NIH HHS/United States
- HL62231/HL/NHLBI NIH HHS/United States
- HL80101/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

