Heterologous expression of human mPRalpha, mPRbeta and mPRgamma in yeast confirms their ability to function as membrane progesterone receptors
- PMID: 18603275
- PMCID: PMC2597464
- DOI: 10.1016/j.steroids.2008.05.003
Heterologous expression of human mPRalpha, mPRbeta and mPRgamma in yeast confirms their ability to function as membrane progesterone receptors
Abstract
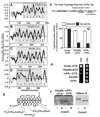
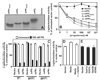
The nuclear progesterone receptor (nPR) mediates many of the physiological effects of progesterone by regulating the expression of genes, however, progesterone also exerts non-transcriptional (non-genomic) effects that have been proposed to rely on a receptor that is distinct from nPR. Several members of the progestin and AdipoQ-Receptor (PAQR) family were recently identified as potential mediators of these non-genomic effects. Membranes from cells expressing these proteins, called mPRalpha, mPRbeta and mPRgamma, were shown to specifically bind progesterone and have G-protein coupled receptor (GPCR) characteristics, although other studies dispute these findings. To clarify the role of these mPRs in non-genomic progesterone signaling, we established an assay for PAQR functional evaluation using heterologous expression in Saccharomyces cerevisiae. Using this assay, we demonstrate unequivocally that mPRalpha, mPRbeta and mPRgamma can sense and respond to progesterone with EC(50) values that are physiologically relevant. Agonist profiles also show that mPRalpha, mPRbeta and mPRgamma are activated by ligands, such as 17alpha-hydroxyprogesterone, that are known to activate non-genomic pathways but not nPR. These results strongly suggest that these receptors may indeed function as the long-sought-after membrane progesterone receptors. Additionally, we show that two uncharacterized PAQRs, PAQR6 and PAQR9, are also capable of responding to progesterone. These mPR-like PAQRs have been renamed as mPRdelta (PAQR6) and mPRvarepsilon (PAQR9). Additional characterization of mPRgamma and mPRalpha indicates that their progesterone-dependent signaling in yeast does not require heterotrimeric G-proteins, thus calling into question the characterization of the mPRs as a novel class of G-protein coupled receptor.
Figures




Similar articles
-
Expression of membrane progestin receptors (mPRs) in the bovine corpus luteum during the estrous cycle and first trimester of pregnancy.Domest Anim Endocrinol. 2018 Apr;63:69-76. doi: 10.1016/j.domaniend.2017.12.004. Epub 2018 Jan 11. Domest Anim Endocrinol. 2018. PMID: 29413904
-
Membrane progesterone receptors β and γ have potential as prognostic biomarkers of endometrial cancer.J Steroid Biochem Mol Biol. 2018 Apr;178:303-311. doi: 10.1016/j.jsbmb.2018.01.011. Epub 2018 Jan 17. J Steroid Biochem Mol Biol. 2018. PMID: 29353001
-
Expression of membrane progestin receptors (mPRs) α, β and γ in the bovine uterus during the oestrous cycle and pregnancy.Theriogenology. 2019 Dec;140:171-179. doi: 10.1016/j.theriogenology.2019.08.028. Epub 2019 Aug 26. Theriogenology. 2019. PMID: 31479833
-
Nonclassical progesterone signalling molecules in the nervous system.J Neuroendocrinol. 2013 Nov;25(11):991-1001. doi: 10.1111/jne.12060. J Neuroendocrinol. 2013. PMID: 23763432 Review.
-
Novel progesterone receptors: neural localization and possible functions.Front Neurosci. 2013 Sep 19;7:164. doi: 10.3389/fnins.2013.00164. Front Neurosci. 2013. PMID: 24065878 Free PMC article. Review.
Cited by
-
Genomic sequence analyses of classical and non-classical lamprey progesterone receptor genes and the inference of homologous gene evolution in metazoans.BMC Evol Biol. 2019 Jul 1;19(1):136. doi: 10.1186/s12862-019-1463-7. BMC Evol Biol. 2019. PMID: 31262250 Free PMC article.
-
The role of adiponectin in reproduction: from polycystic ovary syndrome to assisted reproduction.Fertil Steril. 2010 Nov;94(6):1949-57. doi: 10.1016/j.fertnstert.2010.05.010. Epub 2010 Jun 19. Fertil Steril. 2010. PMID: 20561616 Free PMC article. Review.
-
Characterization of human plasma-derived exosomal RNAs by deep sequencing.BMC Genomics. 2013 May 10;14:319. doi: 10.1186/1471-2164-14-319. BMC Genomics. 2013. PMID: 23663360 Free PMC article.
-
Membrane progestin receptors in the midbrain ventral tegmental area are required for progesterone-facilitated lordosis of rats.Horm Behav. 2013 Aug;64(3):539-45. doi: 10.1016/j.yhbeh.2013.05.012. Epub 2013 Jun 12. Horm Behav. 2013. PMID: 23770270 Free PMC article.
-
PAQR6 Expression Enhancement Suggests a Worse Prognosis in Prostate Cancer Patients.Open Life Sci. 2018 Dec 31;13:511-517. doi: 10.1515/biol-2018-0061. eCollection 2018 Jan. Open Life Sci. 2018. PMID: 33817121 Free PMC article.
References
-
- Kumar R, Thompson EB. The structure of the nuclear hormone receptors. Steroids. 1999;64(5):310–319. - PubMed
-
- Correia JN, Conner SJ, Kirkman-Brown JC. Non-genomic steroid actions in human spermatozoa. Persistent tickling from a laden environment. Semin Reprod Med. 2007;25(3):208–219. - PubMed
-
- Blackmore PF, Fisher JF, Spilman CH, Bleasdale JE. Unusual steroid specificity of the cell surface progesterone receptor on human sperm. Mol Pharmacol. 1996;49(4):727–739. - PubMed
-
- Maller JL. Signal transduction. Fishing at the cell surface. Science. 2003;300(5619):594–595. - PubMed
-
- Boonyaratanakornkit V, Edwards DP. Receptor mechanisms mediating non-genomic actions of sex steroids. Semin Reprod Med. 2007;25(3):139–153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

