Endocytosis of apolipoprotein A-V by members of the low density lipoprotein receptor and the VPS10p domain receptor families
- PMID: 18603531
- PMCID: PMC2533778
- DOI: 10.1074/jbc.M802721200
Endocytosis of apolipoprotein A-V by members of the low density lipoprotein receptor and the VPS10p domain receptor families
Abstract
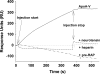
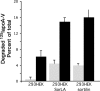
Apolipoprotein A-V (apoA-V) is present in low amounts in plasma and has been found to modulate triacylglycerol levels in humans and in animal models. ApoA-V displays affinity for members of the low density lipoprotein receptor (LDL-R) gene family, known as the classical lipoprotein receptors, including LRP1 and SorLA/LR11. In addition to LDL-A binding repeats, the mosaic receptor SorLA/LR11 also possesses a Vps10p domain. Here we show that apoA-V also binds to sortilin, a receptor from the Vsp10p domain gene family that lacks LDL-A repeats. Binding of apoA-V to sortilin was competed by neurotensin, a ligand that binds specifically to the Vps10p domain. To investigate the biological fate of receptor-bound apoA-V, binding experiments were conducted with cultured human embryonic kidney cells transfected with either SorLA/LR11 or sortilin. Compared with nontransfected cells, apoA-V binding to SorLA/LR11- and sortilin-expressing cells was markedly enhanced. Internalization experiments, live imaging studies, and fluorescence resonance energy transfer analyses demonstrated that labeled apoA-V was rapidly internalized, co-localized with receptors in early endosomes, and followed the receptors through endosomes to the trans-Golgi network. The observed decrease of fluorescence signal intensity as a function of time during live imaging experiments suggested ligand uncoupling in endosomes with subsequent delivery to lysosomes for degradation. This interpretation was supported by experiments with (125)I-labeled apoA-V, demonstrating clear differences in degradation between transfected and nontransfected cells. We conclude that apoA-V binds to receptors possessing LDL-A repeats and Vsp10p domains and that apoA-V is internalized into cells via these receptors. This could be a mechanism by which apoA-V modulates lipoprotein metabolism in vivo.
Figures




References
-
- Pennacchio, L. A., Olivier, M., Hubacek, J. A., Cohen, J. C., Cox, D. R., Fruchart, J. C., Krauss, R. M., and Rubin, E. M. (2001) Science 294169 –173 - PubMed
-
- van Dijk, K. W., Rensen, P.C., Voshol, P. J., and Havekes, L. M. (2004) Curr. Opin. Lipidol. 15239 –246 - PubMed
-
- Hubacek, J. A. (2005) Clin. Chem. Lab. Med. 43897 –902 - PubMed
-
- Talmud, P. J., Hawe, E., Martin, S., Olivier, M., Miller, G. J., Rubin, E. M., Pennacchio, L. A., and Humphries, S. E. (2002) Hum. Mol. Genet. 113039 –3046 - PubMed
-
- Yamada, Y., Kato, K., Hibino, T., Yokoi, K., Matsuo, H., Segawa, T., Watanabe, S., Ichihara, S., Yoshida, H., Satoh, K., and Nozawa, Y. (2007) Atherosclerosis 191298 –304 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

