Targeted deletion of kcne2 impairs ventricular repolarization via disruption of I(K,slow1) and I(to,f)
- PMID: 18603586
- PMCID: PMC2537427
- DOI: 10.1096/fj.08-110171
Targeted deletion of kcne2 impairs ventricular repolarization via disruption of I(K,slow1) and I(to,f)
Abstract
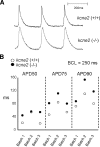
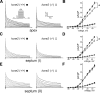
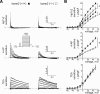
Mutations in human KCNE2, which encodes the MiRP1 potassium channel ancillary subunit, associate with long QT syndrome (LQTS), a defect in ventricular repolarization. The precise cardiac role of MiRP1 remains controversial, in part, because it has marked functional promiscuity in vitro. Here, we disrupted the murine kcne2 gene to define the role of MiRP1 in murine ventricles. kcne2 disruption prolonged ventricular action potential duration (APD), suggestive of reduced repolarization capacity. Accordingly, kcne2 (-/-) ventricles exhibited a 50% reduction in I(K,slow1), generated by Kv1.5--a previously unknown partner for MiRP1. I(to,f), generated by Kv4 alpha subunits, was also diminished, by approximately 25%. Ventricular MiRP1 protein coimmunoprecipitated with native Kv1.5 and Kv4.2 but not Kv1.4 or Kv4.3. Unexpectedly, kcne2 (-/-) ventricular membrane fractions exhibited 50% less mature Kv1.5 protein than wild type, and disruption of Kv1.5 trafficking to the intercalated discs. Consistent with the reduction in ventricular K(+) currents and prolonged ventricular APD, kcne2 deletion lengthened the QT(c) under sevoflurane anesthesia. Thus, targeted disruption of kcne2 has revealed a novel cardiac partner for MiRP1, a novel role for MiRPs in alpha subunit targeting in vivo, and a role for MiRP1 in murine ventricular repolarization with parallels to that proposed for the human heart.
Figures

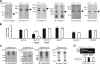

References
-
- McCrossan Z A, Abbott G W. The MinK-related peptides. Neuropharmacology. 2004;47:787–821. - PubMed
-
- Abbott G W, Sesti F, Splawski I, Buck M E, Lehmann M H, Timothy K W, Keating M T, Goldstein S A. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. - PubMed
-
- Reymond A, Marigo V, Yaylaoglu M B, Leoni A, Ucla C, Scamuffa N, Caccioppoli C, Dermitzakis E T, Lyle R, Banfi S, Eichele G, Antonarakis S E, Ballabio A. Human chromosome 21 gene expression atlas in the mouse. Nature. 2001;420:582–586. - PubMed
-
- Chun K R, Koenen M, Katus H A, Zehelein J. Expression of the IKr components KCNH2 (rERG) and KCNE2 (rMiRP1) during late rat heart development. Exp Mol Med. 2004;36:367–371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

