Cell-free co-expression of functional membrane proteins and apolipoprotein, forming soluble nanolipoprotein particles
- PMID: 18603642
- PMCID: PMC2577204
- DOI: 10.1074/mcp.M800191-MCP200
Cell-free co-expression of functional membrane proteins and apolipoprotein, forming soluble nanolipoprotein particles
Abstract
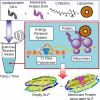
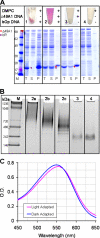
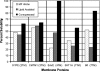
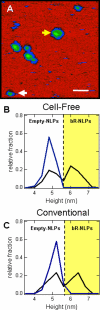
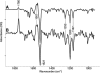
Here we demonstrate rapid production of solubilized and functional membrane protein by simultaneous cell-free expression of an apolipoprotein and a membrane protein in the presence of lipids, leading to the self-assembly of membrane protein-containing nanolipoprotein particles (NLPs). NLPs have shown great promise as a biotechnology platform for solubilizing and characterizing membrane proteins. However, current approaches are limited because they require extensive efforts to express, purify, and solubilize the membrane protein prior to insertion into NLPs. By the simple addition of a few constituents to cell-free extracts, we can produce membrane proteins in NLPs with considerably less effort. For this approach an integral membrane protein and an apolipoprotein scaffold are encoded by two DNA plasmids introduced into cell-free extracts along with lipids. For this study reported here we used plasmids encoding the bacteriorhodopsin (bR) membrane apoprotein and scaffold protein Delta1-49 apolipoprotein A-I fragment (Delta49A1). Cell free co-expression of the proteins encoded by these plasmids, in the presence of the cofactor all-trans-retinal and dimyristoylphosphatidylcholine, resulted in production of functional bR as demonstrated by a 5-nm shift in the absorption spectra upon light adaptation and characteristic time-resolved FT infrared difference spectra for the bR --> M transition. Importantly the functional bR was solubilized in discoidal bR.NLPs as determined by atomic force microscopy. A survey study of other membrane proteins co-expressed with Delta49A1 scaffold protein also showed significantly increased solubility of all of the membrane proteins, indicating that this approach may provide a general method for expressing membrane proteins enabling further studies.
Figures
References
-
- Bayburt, T. H., Carlson, J. W., and Sligar, S. G. ( 1998) Reconstitution and imaging of a membrane protein in a nanometer-size phospholipid bilayer. J. Struct. Biol. 123, 37–44 - PubMed
-
- Bayburt, T. H., Grinkova, Y. V., and Sligar, S. G. ( 2006) Assembly of single bacteriorhodopsin trimers in bilayer nanodiscs. Arch. Biochem. Biophys. 450, 215–222 - PubMed
-
- Leitz, A. J., Bayburt, T. H., Barnakov, A. N., Springer, B. A., and Sligar, S. G. ( 2006) Functional reconstitution of β2-adrenergic receptors utilizing self-assembling Nanodisc technology. BioTechniques 40, 601–602, 604, 606, passim. - PubMed
-
- Bayburt, T. H., Leitz, A. J., Xie, G., Oprian, D. D., and Sligar, S. G. ( 2007) Transducin activation by nanoscale lipid bilayers containing one and two rhodopsins. J. Biol. Chem. 282, 14875–14881 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

