The cardiovascular and pharmacokinetic profile of dofetilide in conscious telemetered beagle dogs and cynomolgus monkeys
- PMID: 18604237
- PMCID: PMC2492096
- DOI: 10.1038/bjp.2008.275
The cardiovascular and pharmacokinetic profile of dofetilide in conscious telemetered beagle dogs and cynomolgus monkeys
Abstract
Background and purpose: The effects of dofetilide were studied in monkeys and dogs. Pharmacokinetic data were generated together with the monitoring of cardiovascular changes in order to compare effects relative to human exposure.
Experimental approach: Beagle dogs and cynomolgus monkeys were telemetered to collect arterial blood pressure, heart rate and ECG for 6 h after selected oral doses of dofetilide. Pharmacokinetic parameters were determined for each dose.
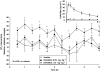
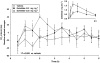
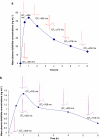
Key results: Dogs: increases in the QT(c) interval reached 56 ms in dogs dosed with 0.3 mg kg(-1) of dofetilide. Premature ventricular contractions and right bundle branch block were evident at this dose, without changes in cardiovascular parameters. The mean C(max) values were 3.35 and 60.15 ng mL(-1) at doses of 0.03 and 0.3 mg kg(-1), respectively. Monkeys: increases in QT(c) intervals reached 40-50 ms after 0.03 mg kg(-1). T-wave changes were observed after 0.03 mg kg(-1) without changes in cardiovascular parameters. The mean C(max) values following oral doses of 0.01 and 0.03 mg kg(-1) were 0.919 ng mL(-1) and 1.85 ng mL(-1), respectively.
Conclusions and implications: Despite dofetilide exposure comparable to that in humans, QT(c) responses in dogs were greater than those reported in humans. A comparable human dose used in the monkey achieved only half of the exposure but was associated with twofold greater increases in QT(c). Our data support the view that safety risk assessments of new drugs in animal models should ensure that the clinical therapeutic range of exposure is achieved and any untoward effects interpreted accordingly.
Figures
References
-
- Anon ICH S7A Safety pharmacology studies for human pharmaceuticals, implemented by MHLW, CPMP, FDA. Federal Register. 2001;66:36791–36792. - PubMed
-
- Anon ICHS7B Guidance on safety pharmacology studies for assessing the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals. 2005. - PubMed
-
- Belardinelli L, Antzelevitch C, Vos MA. Assessing predictors of drug-induced torsade de pointes. Trends Pharmacol Sci. 2003;24:619–625. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

