Estimating the efficiency of benzodiazepines on GABA(A) receptors comprising gamma1 or gamma2 subunits
- PMID: 18604239
- PMCID: PMC2451336
- DOI: 10.1038/bjp.2008.271
Estimating the efficiency of benzodiazepines on GABA(A) receptors comprising gamma1 or gamma2 subunits
Abstract
Background and purpose: Heterologous expression of alpha1, beta2 and gamma2S(gamma1) subunits produces a mixed population of GABA(A) receptors containing alpha1beta2 or alpha1beta2gamma2S(gamma1) subunits. GABA sensitivity (lower in receptors containing gamma1 or gamma2S subunits) and the potentiation of GABA-activated chloride currents (I(GABA)) by benzodiazepines (BZDs) are dependent on gamma2S(gamma1) incorporation. A variable gamma subunit incorporation may affect the estimation of I(GABA) potentiation by BZDs. We propose an approach for estimation of BZD efficiency that accounts for mixed population of alpha1beta2 and alpha1beta2gamma2S(gamma1) receptors.
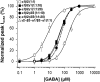
Experimental approach: We investigated the relation between GABA sensitivity (EC50) and BZD modulation by analysing triazolam-, clotiazepam- and midazolam-induced potentiation of I(GABA) in Xenopus oocytes under two-microelectrode voltage clamp.
Key results: Plotting EC50 versus BZD-induced shifts of GABA concentration-response curves (DeltaEC50(BZD)) of oocytes injected with different amounts of alpha1, beta2 and gamma2S(gamma1) cRNA (1:1:1-1:1:10) revealed a linear regression between gamma2S(gamma1)-mediated reduction of GABA sensitivity (EC50) and DeltaEC50(BZD). The slope factors of the regression were always higher for oocytes expressing alpha1beta2gamma1 subunit receptors (1.8 +/- 0.1 (triazolam), 1.6 +/- 0.1 (clotiazepam), 2.3 +/- 0.2 (midazolam)) than for oocytes expressing alpha1beta2gamma2S receptors (1.4 +/- 0.1 (triazolam), 1.4 +/- 0.1 (clotiazepam), 1.3 +/- 0.1 (midazolam)). Mutant GABA(A) receptors (alpha1beta2-R207Cgamma2S) with lower GABA sensitivity showed higher drug efficiencies (slope factors=1.1 +/- 0.1 (triazolam), 1.1 +/- 0.1 (clotiazepam), 1.2 +/- 0.1 (midazolam)).
Conclusions and implications: Regression analysis enabled the estimation of BZD efficiency when variable mixtures of alpha1beta2 and alpha1beta2gamma2S(gamma1) receptors are expressed and provided new insights into the gamma2S(gamma1) dependency of BZD action.
Figures





References
-
- Akabas MH. GABAA receptor structure-function studies: a reexamination in light of new acetylcholine receptor structures. Int Rev Neurobiol. 2004;62:1–43. - PubMed
-
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the beta-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. - PubMed
-
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, et al. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

