Quantification of protein phosphorylation by liquid chromatography-mass spectrometry
- PMID: 18605695
- PMCID: PMC3050605
- DOI: 10.1021/ac800337v
Quantification of protein phosphorylation by liquid chromatography-mass spectrometry
Abstract
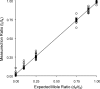
The identification and quantification of specific phosphorylation sites within a protein by mass spectrometry has proved challenging when measured from peptides after protein digestion because each peptide has a unique ionization efficiency that alters with modification, such as phosphorylation, and because phosphorylation can alter cleavage by trypsin, shifting peptide distribution. In addition, some phosphorylated peptides generated by tryptic digest are small and hydrophilic and, thus, are not retained well on commonly used C18 columns. We have developed a novel C-terminal peptide (2)H-labeling derivatization strategy and a mass balance approach to quantify phosphorylation. We illustrate the application of our method using electrospray ionization liquid chromatography-mass spectrometry by quantifying phosphorylation of troponin I with protein kinase A and protein kinase C. The method also improves the retention and elution of hydrophilic peptides. The method defines phosphorylation without having to measure the phosphorylated peptides directly or being affected by variable miscleavage. Measurement of phosphorylation is shown to be linear (relative standard error <5%) with a detection limit of <10%.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

