IL-17A inhibits the expansion of IL-17A-producing T cells in mice through "short-loop" inhibition via IL-17 receptor
- PMID: 18606690
- PMCID: PMC2586908
- DOI: 10.4049/jimmunol.181.2.1357
IL-17A inhibits the expansion of IL-17A-producing T cells in mice through "short-loop" inhibition via IL-17 receptor
Abstract
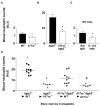
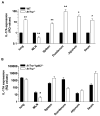
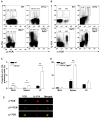
IL-23 and IL-17A regulate granulopoiesis through G-CSF, the main granulopoietic cytokine. IL-23 is secreted by activated macrophages and dendritic cells and promotes the expansion of three subsets of IL-17A-expressing neutrophil-regulatory T (Tn) cells; CD4(-)CD8(-)alphabeta(low), CD4(+)CD8(-)alphabeta(+) (Th17), and gammadelta(+) T cells. In this study, we investigate the effects of IL-17A on circulating neutrophil levels using IL-17R-deficient (Il17ra(-/-)) mice and Il17ra(-/-)Itgb2(-/-) mice that lack both IL-17R and all four beta(2) integrins. IL-17R deficiency conferred a reduction in neutrophil numbers and G-CSF levels, as did Ab blockade against IL-17A in wild-type mice. Bone marrow transplantation revealed that IL-17R expression on nonhemopoietic cells had the greatest effects on regulating blood neutrophil counts. Although circulating neutrophil numbers were reduced, IL-17A expression, secretion, and the number of IL-17A-producing Tn cells were elevated in Il17ra(-/-) and Il17ra(-/-)Itgb2(-/-) mice, suggesting a negative feedback effect through IL-17R. The negative regulation of IL-17A-producing T cells and IL-17A and IL-17F gene expression through the interactions of IL-17A or IL-17F with IL-17R was confirmed in splenocyte cultures in vitro. We conclude that IL-17A regulates blood neutrophil counts by inducing G-CSF production mainly in nonhemopoietic cells. IL-17A controls the expansion of IL-17A-producing Tn cell populations through IL-17R.
Figures




References
-
- Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. - PubMed
-
- Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–1914. - PubMed
-
- Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of Immune Induction of Collagen-Induced Arthritis in IL-17-Deficient Mice. J Immunol. 2003;171:6173–6177. - PubMed
-
- Miossec P. IL-17 in rheumatoid arthritis: a new target for treatment or just another cytokine? Joint Bone Spine. 2004;71:87–90. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

