CD11c expression identifies a population of extrafollicular antigen-specific splenic plasmablasts responsible for CD4 T-independent antibody responses during intracellular bacterial infection
- PMID: 18606692
- PMCID: PMC2645789
- DOI: 10.4049/jimmunol.181.2.1375
CD11c expression identifies a population of extrafollicular antigen-specific splenic plasmablasts responsible for CD4 T-independent antibody responses during intracellular bacterial infection
Abstract
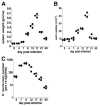
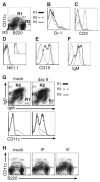
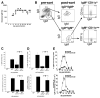
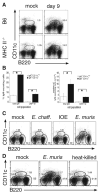
Although T-independent immunity is known to be generated against bacterial capsular and cell wall polysaccharides expressed by a number of bacterial pathogens, it has not been studied in depth during intracellular bacterial infections. Our previous study demonstrated that Ehrlichia muris, an obligate intracellular tick-borne pathogen, generates protective classical TI responses in CD4 T cell-deficient C57BL/6 mice. We found that E. muris T-independent immunity is accompanied by the expansion of a very large extrafollicular spleen population of CD11c(low)-expressing plasmablasts that exhibit characteristics of both B-1 and marginal zone B cells. The plasmablasts comprised up to 15% of the total spleen lymphocytes and approximately 70% of total spleen IgM(high)IgD(low) cells during peak infection in both wild-type and MHC class II-deficient mice. The CD11c(low) cells exhibited low surface expression of B220, CD19, and CD1d, high expression of CD11b, CD43, but did not express CD5. Approximately 50% of the CD11c(low) cells also expressed CD138. In addition to CD11b and CD11c, the plasmablasts expressed the beta(1) (CD29) and alpha4 (CD49d) integrins, as well as the chemokine receptor CXCR4, molecules which may play roles in localizing the B cells extrafollicular region of the spleen. During peak infection, the CD11c(low) cells accounted for the majority of the IgM-producing splenic B cells and nearly all of the E. muris outer membrane protein-specific IgM-secreting cells. Thus, during this intracellular bacterial infection, CD11c expression identifies a population of Ag-specific spleen plasmablasts responsible for T-independent Ab production.
Conflict of interest statement
Figures



References
-
- Martin F, Kearney JF. B1 cells: similarities and differences with other B cell subsets. Curr Opin Immunol. 2001;13:195–201. - PubMed
-
- Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. - PubMed
-
- Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. - PubMed
-
- Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. 2000;176:154–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

