Hepatitis B virus X protein via the p38MAPK pathway induces E2F1 release and ATR kinase activation mediating p53 apoptosis
- PMID: 18606816
- PMCID: PMC2533071
- DOI: 10.1074/jbc.M801934200
Hepatitis B virus X protein via the p38MAPK pathway induces E2F1 release and ATR kinase activation mediating p53 apoptosis
Abstract
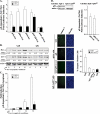
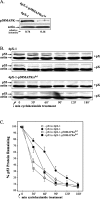
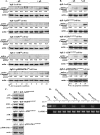
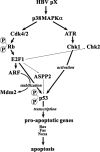
Hepatitis B virus (HBV) X protein (pX) is implicated in hepatocellular carcinoma (HCC) pathogenesis by an unknown mechanism. Deletions or mutations of genes involved in the p53 pathway are often associated with HBV-mediated HCC, indicating rescue from p53 apoptosis is a likely mechanism in HBV-HCC pathogenesis. Herein, we determined the mechanism by which pX sensitizes hepatocytes to p53-mediated apoptosis. Although it is well established that the Rb/E2F/ARF pathway stabilizes p53, and the DNA damage-activated ATM/ATR kinases activate p53, the mechanism that coordinates these two pathways has not been determined. We demonstrate that the p38MAPK pathway activated by pX serves this role in p53 apoptosis. Specifically, the activated p38MAPK pathway stabilizes p53 via E2F1-mediated ARF expression, and also activates the transcriptional function of p53 by activating ATR. Knockdown of p53, E2F1, ATR, or p38MAPKalpha abrogates pX-mediated apoptosis, demonstrating that E2F1, ATR, and p38MAPKalpha are all essential in p53 apoptosis in response to pX. Specifically, in response to pX expression, the p38MAPK pathway activates Cdk4 and Cdk2, leading to phosphorylation of Rb, release of E2F1, and transcription of ARF. The p38MAPK pathway also activates ATR, leading to phosphorylation of p53 on Ser-18 and Ser-23, transcription of pro-apoptotic genes Bax, Fas, and Noxa, and apoptosis. In conclusion, pX sensitizes hepatocytes to p53 apoptosis via activation of the p38MAPK pathway, which couples p53 stabilization and p53 activation, by E2F1 induction and ATR activation, respectively.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

