Interferon autoantibodies associated with AIRE deficiency decrease the expression of IFN-stimulated genes
- PMID: 18606876
- PMCID: PMC2577576
- DOI: 10.1182/blood-2008-03-144634
Interferon autoantibodies associated with AIRE deficiency decrease the expression of IFN-stimulated genes
Abstract
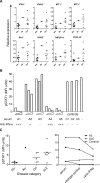
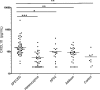
Neutralizing autoantibodies to type I, but not type II, interferons (IFNs) are found at high titers in almost every patient with autoimmune polyendocrinopathy candidiasis ectodermal dystrophy (APECED), a disease caused by AIRE gene mutations that lead to defects in thymic T-cell selection. Combining genome-wide expression array with real time RT-PCR assays, we here demonstrate that antibodies against IFN-alpha cause highly significant down-regulation of interferon-stimulated gene expression in cells from APECED patients' blood by blocking their highly dilute endogenous IFNs. This down-regulation was lost progressively as these APECED cells matured in cultures without neutralizing autoantibodies. Most interestingly, a rare APECED patient with autoantibodies to IFN-omega but not IFN-alpha showed a marked increase in expression of the same interferon-stimulated genes. We also report unexpected increases in serum CXCL10 levels in APECED. Our results argue that the breakdown of tolerance to IFNs in AIRE deficiency is associated with impaired responses to them in thymus, and highlight APECED as another autoimmune disease with associated dysregulation of IFN activity.
Figures




References
-
- Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. - PubMed
-
- Baccala R, Kono DH, Theofilopoulos AN. Interferons as pathogenic effectors in autoimmunity. Immunol Rev. 2005;204:9–26. - PubMed
-
- Saksela E, Virtanen I, Hovi T, Secher DS, Cantell K. Monocyte is the main producer of human leukocyte alpha interferons following Sendai virus induction. Prog Med Virol. 1984;30:78–86. - PubMed
-
- Liu YJIPC: professional type 1 interferon-producing cells and plasmacytoid dendritic cell precursors. Annu Rev Immunol. 2005;23:275–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

