The paradoxical effects of using antiretroviral-based microbicides to control HIV epidemics
- PMID: 18606986
- PMCID: PMC2447864
- DOI: 10.1073/pnas.0711813105
The paradoxical effects of using antiretroviral-based microbicides to control HIV epidemics
Erratum in
- Proc Natl Acad Sci U S A. 2009 Apr 21;106(16):6879
Abstract
Vaginal microbicides, designed to prevent HIV infection in women, are one of the most promising biomedical interventions. Clinical trials of second-generation microbicides have begun; if shown to be effective, they could be licensed within 5-10 years. Because these microbicides contain antiretrovirals (ARVs), they could be highly effective. However, there is concern that, if used by HIV-positive women, ARV resistance may evolve. By analyzing a mathematical model, we find that adherence could have both beneficial and detrimental effects on trial outcomes. Most importantly, we show that planned trial designs could mask resistance risks and therefore enable high-risk microbicides to pass clinical testing. We then parameterize a transmission model using epidemiological, clinical, and behavioral data to predict the consequences of wide-scale usage of high-risk microbicides in a heterosexual population. Surprisingly, we show that reducing a participant's risk of resistance during a trial could lead to unexpectedly high rates of resistance afterward when microbicides are used in public health interventions. We also find that, paradoxically, although microbicides will be used by women to protect themselves against infection, they could provide greater benefit to men. More infections in men than in women will be prevented if there is a high probability that ARVs are systemically absorbed, microbicides are less than approximately 50% effective, and/or adherence is less than approximately 60%. Men will always benefit more than women in terms of infections prevented per resistant case; but this advantage decreases as the relative fitness of drug-resistant strains increases. Interventions that use ARV-based microbicides could have surprising consequences.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
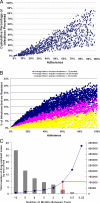
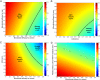
Comment in
-
Predicting the unpredictable real-world impact of ARV-based microbicides.Proc Natl Acad Sci U S A. 2008 Nov 4;105(44):E73; author reply E74. doi: 10.1073/pnas.0807633105. Epub 2008 Oct 30. Proc Natl Acad Sci U S A. 2008. PMID: 18974215 Free PMC article. No abstract available.
References
-
- Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nat Rev Microbiol. 2003;1:25–34. - PubMed
-
- Veazey RS, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med. 2003;9:343–346. - PubMed
-
- Joint United Nations Programme on HIV/AIDS (UNAIDS) Report on the Global AIDS Epidemic. Geneva: Joint United Nations Programme on HIV/AIDS; 2006. - PubMed
-
- D'Cruz OJ, Uckun FM. Clinical development of microbicides for the prevention of HIV infection. Curr Pharm Des. 2004;10:315–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

