Mucosal penetration primes Vibrio cholerae for host colonization by repressing quorum sensing
- PMID: 18606988
- PMCID: PMC2474479
- DOI: 10.1073/pnas.0802241105
Mucosal penetration primes Vibrio cholerae for host colonization by repressing quorum sensing
Abstract
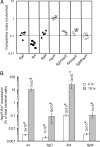
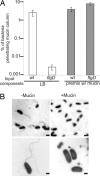
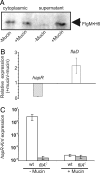
To successfully infect a host and cause the diarrheal disease cholera, Vibrio cholerae must penetrate the intestinal mucosal layer and express virulence genes. Previous studies have demonstrated that the transcriptional regulator HapR, which is part of the quorum sensing network in V. cholerae, represses the expression of virulence genes. Here, we show that hapR expression is also modulated by the regulatory network that governs flagellar assembly. Specifically, FliA, which is the alternative sigma-factor (sigma(28)) that activates late-class flagellin genes in V. cholerae, represses hapR expression. In addition, we show that mucin penetration by V. cholerae is sufficient to break flagella and so cause the secretion of FlgM, the anti-sigma factor that inhibits FliA activity. During initial colonization of host intestinal tissue, hapR expression is repressed because of low cell density. However, full repression of hapR expression does not occur in fliA mutants, which results in attenuated colonization. Our results suggest that V. cholerae uses flagellar machinery to sense particular intestinal signals before colonization and enhance the expression of virulence genes by modulating the output of quorum sensing signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Reidl J, Klose KE. Vibrio cholerae and cholera: out of the water and into the host. FEMS Microbiol Rev. 2002;26:125–139. - PubMed
-
- Waters CM, Bassler BL. Quorum sensing: Cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. - PubMed
-
- Higgins DA, et al. The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature. 2007;450:883–886. - PubMed
-
- Lenz DH, et al. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell. 2004;118:69–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

