Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1
- PMID: 18606998
- PMCID: PMC2474477
- DOI: 10.1073/pnas.0803223105
Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1
Abstract
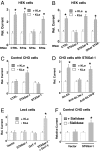
Klotho is a mammalian senescence-suppression protein that has homology with glycosidases. The extracellular domain of Klotho is secreted into urine and blood and may function as a humoral factor. Klotho-deficient mice have accelerated aging and imbalance of ion homeostasis. Klotho treatment increases cell-surface abundance of the renal epithelial Ca(2+) channel TRPV5 by modifying its N-linked glycans. However, the precise sugar substrate and mechanism for regulation by Klotho is not known. Here, we report that the extracellular domain of Klotho activates plasma-membrane resident TRPV5 through removing terminal sialic acids from their glycan chains. Removal of sialic acids exposes underlying disaccharide galactose-N-acetylglucosamine, a ligand for a ubiquitous galactoside-binding lectin galectin-1. Binding to galectin-1 lattice at the extracellular surface leads to accumulation of functional TRPV5 on the plasma membrane. Knockdown of beta-galactoside alpha2,6-sialyltransferase (ST6Gal-1) by RNA interference, but not other sialyltransferases, in a human cell line prevents the regulation by Klotho. Moreover, the regulation by Klotho is absent in a hamster cell line that lacks endogenous ST6Gal-1, but is restored by forced expression of recombinant ST6Gal-1. Thus, Klotho participates in specific removal of alpha2,6-linked sialic acids and regulates cell surface retention of TRPV5 through this activity. This action of Klotho represents a novel mechanism for regulation of the activity of cell-surface glycoproteins and likely contributes to maintenance of calcium balance by Klotho.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kuro-o M, et al. Mutation of the mouse klotho gene leads to a syndrome resembling aging. Nature. 1997;390:45–51. - PubMed
-
- Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418. - PubMed
-
- Ogata N, et al. Association of klotho gene polymorphism with bone density and spondylosis of the lumbar spine in postmenopausal women. Bone. 2002;31:37–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

