Ribophorin I regulates substrate delivery to the oligosaccharyltransferase core
- PMID: 18607003
- PMCID: PMC2443820
- DOI: 10.1073/pnas.0711846105
Ribophorin I regulates substrate delivery to the oligosaccharyltransferase core
Abstract
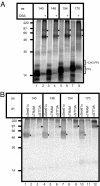
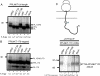
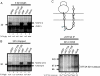
Protein N-glycosylation is widespread among biological systems, and the fundamental process of transferring a lipid-linked glycan to suitable asparagine residues of newly synthesized proteins occurs in both prokaryotes and eukaryotes. The core reaction is mediated by Stt3p family members, and in many organisms this component alone is sufficient to constitute the so called oligosaccharyltransferase (OST). However, eukaryotes typically have a more elaborate OST with several additional subunits of poorly defined function. In the mammalian OST complex one such subunit, ribophorin I, is proposed to facilitate the N-glycosylation of certain precursors during their biogenesis at the endoplasmic reticulum. Here, we use cell culture models to show that ribophorin I depletion results in substrate-specific defects in N-glycosylation, clearly establishing a defined physiological role for ribophorin I. To address the molecular mechanism of ribophorin I function, a cross-linking approach was used to explore the environment of nascent glycoproteins during the N-glycosylation reaction. We show for the first time that ribophorin I can regulate the delivery of precursor proteins to the OST complex by capturing substrates and presenting them to the catalytic core.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Structural Insight into the Mechanism of N-Linked Glycosylation by Oligosaccharyltransferase.Biomolecules. 2020 Apr 17;10(4):624. doi: 10.3390/biom10040624. Biomolecules. 2020. PMID: 32316603 Free PMC article. Review.
-
Studying endoplasmic reticulum function in vitro using siRNA.Methods Mol Biol. 2010;619:389-402. doi: 10.1007/978-1-60327-412-8_23. Methods Mol Biol. 2010. PMID: 20419423
-
OST4 is a subunit of the mammalian oligosaccharyltransferase required for efficient N-glycosylation.J Cell Sci. 2013 Jun 15;126(Pt 12):2595-606. doi: 10.1242/jcs.115410. Epub 2013 Apr 19. J Cell Sci. 2013. PMID: 23606741 Free PMC article.
-
Ribophorin I associates with a subset of membrane proteins after their integration at the sec61 translocon.J Biol Chem. 2005 Feb 11;280(6):4195-206. doi: 10.1074/jbc.M410329200. Epub 2004 Nov 19. J Biol Chem. 2005. PMID: 15556939
-
An evolving view of the eukaryotic oligosaccharyltransferase.Glycobiology. 2006 Apr;16(4):47R-62R. doi: 10.1093/glycob/cwj066. Epub 2005 Nov 29. Glycobiology. 2006. PMID: 16317064 Review.
Cited by
-
Oligosaccharyltransferase structures provide novel insight into the mechanism of asparagine-linked glycosylation in prokaryotic and eukaryotic cells.Glycobiology. 2019 Apr 1;29(4):288-297. doi: 10.1093/glycob/cwy093. Glycobiology. 2019. PMID: 30312397 Free PMC article. Review.
-
Platelets and Defective N-Glycosylation.Int J Mol Sci. 2020 Aug 6;21(16):5630. doi: 10.3390/ijms21165630. Int J Mol Sci. 2020. PMID: 32781578 Free PMC article. Review.
-
Intracellular lectins are involved in quality control of glycoproteins.Proc Jpn Acad Ser B Phys Biol Sci. 2014;90(2):67-82. doi: 10.2183/pjab.90.67. Proc Jpn Acad Ser B Phys Biol Sci. 2014. PMID: 24522156 Free PMC article. Review.
-
Dysregulation of N-glycosylation by Rpn1 knockout in spermatocytes induces male infertility via endoplasmic reticulum stress in mice.Int J Biol Sci. 2025 Mar 3;21(5):2360-2379. doi: 10.7150/ijbs.106468. eCollection 2025. Int J Biol Sci. 2025. PMID: 40083683 Free PMC article.
-
N-linked protein glycosylation in the endoplasmic reticulum.Cold Spring Harb Perspect Biol. 2013 Aug 1;5(8):a013359. doi: 10.1101/cshperspect.a013359. Cold Spring Harb Perspect Biol. 2013. PMID: 23751184 Free PMC article. Review.
References
-
- Kelleher DJ, Gilmore R. An evolving view of the eukaryotic oligosaccharyltransferase. Glycobiology. 2006;16:47R–62R. - PubMed
-
- Freeze HH, Aebi M. Altered glycan structures: The molecular basis of congenital disorders of glycosylation. Curr Opin Struct Biol. 2005;15:490–498. - PubMed
-
- Kelleher DJ, Karaoglu D, Mandon EC, Gilmore R. Oligosaccharyltransferase isoforms that contain different catalytic STT3 subunits have distinct enzymatic properties. Mol Cell. 2003;12:101–111. - PubMed
-
- Shibatani T, David LL, McCormack AL, Frueh K, Skach WR. Proteomic analysis of mammalian oligosaccharyltransferase reveals multiple subcomplexes that contain Sec61, TRAP, and two potential new subunits. Biochemistry. 2005;44:5982–5992. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

