Estrogen effects on epithelial proliferation and benign proliferative lesions in the postmenopausal primate mammary gland
- PMID: 18607345
- PMCID: PMC2691895
- DOI: 10.1038/labinvest.2008.64
Estrogen effects on epithelial proliferation and benign proliferative lesions in the postmenopausal primate mammary gland
Abstract
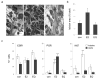
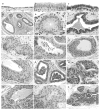
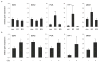
Proliferative lesions of the mammary gland are risk markers and potential precursors for the development of breast cancer in postmenopausal women. In this study we evaluated mammary epithelial proliferation and proliferative lesions in a group of 63 aged postmenopausal macaques randomized by social group to receive one of three experimental diets for 8 months: (1) control; (2) control with 17beta-estradiol (E2) at the human equivalent dose of 1.0 mg per day; and (3) control with the soy phytoestrogen equol (EQ) at the human equivalent dose of 105 mg per day. In normal mammary epithelium, treatment with E2 but not EQ resulted in greater proliferation, epithelial area, and progesterone receptor expression (P<0.05 for all). Mammary lesions included columnar cell change (26/63), columnar cell hyperplasia with and without atypia (13/63), atypical ductal hyperplasia (6/63), and atypical lobular hyperplasia (3/63). Lesions were most common within terminal ductal lobular units. The prevalence of columnar cell hyperplasia (total and atypical cases) was higher in animals treated with E2 compared to control (P<0.05 for both). Compared to normal mammary epithelium, columnar cell lesions (CCLs) showed greater constitutive expression of estrogen receptor-alpha across all groups (P<0.001) and greater expression of progesterone receptor in response to E2 (P<0.01). Independent of treatment, animals with CCLs on histology had greater gene expression of estrogen receptor-alpha and markers of estrogen receptor activity (trefoil factor 1) and proliferation (gene for Ki67 antigen) at a site contralateral to the CCL (P<0.05 for all). These findings demonstrate that the terminal ductal lobular units of the postmenopausal mammary gland contain morphologically distinct cell populations that may hyperrespond to E2 exposure, resulting in specific types of hyperplastic lesions.
Figures
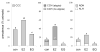

Similar articles
-
[Effects of estrogen monotherapy on breast tissue in the primate model (Macaca fascicularis)].Zentralbl Gynakol. 1997;119(12):607-10. Zentralbl Gynakol. 1997. PMID: 9483811 German.
-
Proliferative effects of combination estrogen and progesterone replacement therapy on the normal postmenopausal mammary gland in a murine model.Am J Obstet Gynecol. 2001 Feb;184(3):340-9. doi: 10.1067/mob.2001.110447. Am J Obstet Gynecol. 2001. PMID: 11228484
-
Progestin effects on cell proliferation pathways in the postmenopausal mammary gland.Breast Cancer Res. 2013;15(4):R62. doi: 10.1186/bcr3456. Breast Cancer Res. 2013. PMID: 23938070 Free PMC article.
-
NTP Research Report on the CLARITY-BPA Core Study: A Perinatal and Chronic Extended-Dose-Range Study of Bisphenol A in Rats: Research Report 9 [Internet].Research Triangle Park (NC): National Toxicology Program; 2018 Sep. Research Triangle Park (NC): National Toxicology Program; 2018 Sep. PMID: 31305969 Free Books & Documents. Review.
-
Hormonal effects on the mammary gland of postmenopausal nonhuman primates.Breast Dis. 2005-2006;24:59-70. doi: 10.3233/bd-2006-24105. Breast Dis. 2005. PMID: 16917139 Review.
Cited by
-
Soy and Isoflavones: Revisiting Their Potential Links to Breast Cancer Risk.Nutrients. 2025 Aug 13;17(16):2621. doi: 10.3390/nu17162621. Nutrients. 2025. PMID: 40871649 Free PMC article. Review.
-
Effects of bazedoxifene acetate with and without conjugated equine estrogens on the breast of postmenopausal monkeys.Menopause. 2012 Nov;19(11):1242-52. doi: 10.1097/GME.0b013e318252e46d. Menopause. 2012. PMID: 23103754 Free PMC article.
-
Developmental and Reproductive Effects of SE5-OH: An Equol-Rich Soy-Based Ingredient.J Toxicol. 2009;2009:307618. doi: 10.1155/2009/307618. Epub 2008 Dec 15. J Toxicol. 2009. PMID: 20107584 Free PMC article.
-
International Harmonization of Nomenclature and Diagnostic Criteria (INHAND): Non-proliferative and Proliferative Lesions of the Non-human Primate (M. fascicularis).J Toxicol Pathol. 2021;34(3 Suppl):1S-182S. doi: 10.1293/tox.34.1S. Epub 2021 Sep 28. J Toxicol Pathol. 2021. PMID: 34712008 Free PMC article. Review.
-
Mammary gland and endometrial effects of testosterone in combination with oral estradiol and progesterone.Menopause. 2009 May-Jun;16(3):466-76. doi: 10.1097/gme.0b013e318191747a. Menopause. 2009. PMID: 19265727 Free PMC article.
References
-
- Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. - PubMed
-
- Santen RJ, Mansel R. Benign breast disorders. N Engl J Med. 2005;353:275–285. - PubMed
-
- Hartmann LC, Sellers TA, Frost MH, et al. Benign breast disease and the risk of breast cancer. N Engl J Med. 2005;353:229–237. - PubMed
-
- Carter CL, Corle DK, Micozzi MS, et al. A prospective study of the development of breast cancer in 16,692 women with benign breast disease. Am J Epidemiol. 1988;128:467–477. - PubMed
-
- London SJ, Connolly JL, Schnitt SJ, et al. A prospective study of benign breast disease and the risk of breast cancer. JAMA. 1992;267:941–944. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

