Biomolecular engineering by combinatorial design and high-throughput screening: small, soluble peptides that permeabilize membranes
- PMID: 18611015
- PMCID: PMC2582735
- DOI: 10.1021/ja8017863
Biomolecular engineering by combinatorial design and high-throughput screening: small, soluble peptides that permeabilize membranes
Abstract
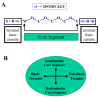
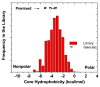
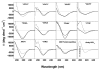
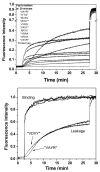
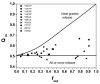
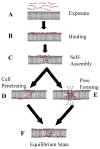
Rational design and engineering of membrane-active peptides remains a largely unsatisfied goal. We have hypothesized that this is due, in part, to the fact that some membrane activities, such as permeabilization, are not dependent on specific amino acid sequences or specific three-dimensional peptide structures. Instead they depend on interfacial activity: the ability of a molecule to partition into the membrane-water interface and to alter the packing and organization of lipids. Here we test that idea by taking a nonclassical approach to biomolecular engineering and design of membrane-active peptides. A 16,384-member rational combinatorial peptide library, containing peptides of 9-15 amino acids in length, was screened for soluble members that permeabilize phospholipid membranes. A stringent, two-phase, high-throughput screen was used to identify 10 unique peptides that had potent membrane-permeabilizing activity but were also water soluble. These rare and uniquely active peptides do not share any particular sequence motif, peptide length, or net charge, but instead they share common compositional features, secondary structure, and core hydrophobicity. We show that they function by a common mechanism that depends mostly on interfacial activity and leads to transient pore formation. We demonstrate here that composition-space peptide libraries coupled with function-based high-throughput screens can lead to the discovery of diverse, soluble, and highly potent membrane-permeabilizing peptides.
Figures


References
-
- White SH, Wimley WC. Membrane protein folding and stability: physical principles. Annu Rev Biophys Biomol Struct. 1999;28:319–365. - PubMed
-
- White SH, Wimley WC. Hydrophobic interactions of peptides with membrane interfaces. Biochim Biophys Acta. 1998;1376(3):339–352. - PubMed
-
- Nguyen LT, Schibli DJ, Vogel HJ. Structural studies and model membrane interactions of two peptides derived from bovine lactoferricin. J Pept Sci. 2005;11(7):379–389. - PubMed
-
- Kondejewski LH, Jelokhani-Niaraki M, Farmer SW, Lix B, Kay CM, Sykes BD, Hancock RE, Hodges RS. Dissociation of antimicrobial and hemolytic activities in cyclic peptide diastereomers by systematic alterations in amphipathicity. J Biol Chem. 1999;274(19):13181–13192. - PubMed
-
- Shai Y, Oren Z. From “carpet” mechanism to de-novo designed diastereomeric cell-selective antimicrobial peptides. Peptides. 2001;22(10):1629–1641. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

