Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage
- PMID: 18611145
- PMCID: PMC2656914
- DOI: 10.1089/ten.tea.2007.0222
Transient exposure to transforming growth factor beta 3 under serum-free conditions enhances the biomechanical and biochemical maturation of tissue-engineered cartilage
Abstract
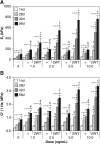
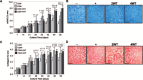
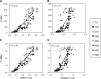
A goal of cartilage tissue engineering is the production of cell-laden constructs possessing sufficient mechanical and biochemical features to enable native tissue function. This study details a systematic characterization of a serum-free (SF) culture methodology employing transient growth factor supplementation to promote robust maturation of tissue-engineered cartilage. Bovine chondrocyte agarose hydrogel constructs were cultured under free-swelling conditions in serum-containing or SF medium supplemented continuously or transiently with varying doses of transforming growth factor beta 3 (TGF-beta3). Constructs were harvested weekly or bi-weekly and assessed for mechanical and biochemical properties. Transient exposure (2 weeks) to low concentrations (2.5-5 ng/mL) of TGF-beta3 in chemically defined medium facilitated robust and highly reproducible construct maturation. Constructs receiving transient TGF-beta3 exposure achieved native tissue levels of compressive modulus (0.8 MPa) and proteoglycan content (6-7% of wet weight) after less than 2 months of in vitro culture. This maturation response was far superior to that observed after continuous growth factor supplementation or transient TGF-beta3 treatment in the presence of serum. These findings represent a significant advance in developing an ex vivo culture methodology to promote production of clinically relevant and mechanically competent tissue-engineered cartilage constructs for implantation to repair damaged articular surfaces.
Figures




References
-
- Guilak F. Sah R.L. Setton L.A. Physical Regulation of Cartilage Metabolism, in Basic Orthopaedic Biomechanics. In: Mow V.C., editor; Hayes W.C., editor. Lippincott-Raven; Philadelphia: 1997. pp. 179–207.
-
- Vunjak-Novakovic G. Obradovic B. Martin I. Bursac P.M. Langer R. Freed L.E. Dynamic cell seeding of polymer scaffolds for cartilage tissue engineering. Biotechnol Prog. 1998;14:193. - PubMed
-
- Buschmann M.D. Gluzband Y.A. Grodzinsky A.J. Hunziker E.B. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108((Pt 4)):1497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

