Large scale variation in Enterococcus faecalis illustrated by the genome analysis of strain OG1RF
- PMID: 18611278
- PMCID: PMC2530867
- DOI: 10.1186/gb-2008-9-7-r110
Large scale variation in Enterococcus faecalis illustrated by the genome analysis of strain OG1RF
Abstract
Background: Enterococcus faecalis has emerged as a major hospital pathogen. To explore its diversity, we sequenced E. faecalis strain OG1RF, which is commonly used for molecular manipulation and virulence studies.
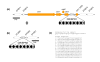
Results: The 2,739,625 base pair chromosome of OG1RF was found to contain approximately 232 kilobases unique to this strain compared to V583, the only publicly available sequenced strain. Almost no mobile genetic elements were found in OG1RF. The 64 areas of divergence were classified into three categories. First, OG1RF carries 39 unique regions, including 2 CRISPR loci and a new WxL locus. Second, we found nine replacements where a sequence specific to V583 was substituted by a sequence specific to OG1RF. For example, the iol operon of OG1RF replaces a possible prophage and the vanB transposon in V583. Finally, we found 16 regions that were present in V583 but missing from OG1RF, including the proposed pathogenicity island, several probable prophages, and the cpsCDEFGHIJK capsular polysaccharide operon. OG1RF was more rapidly but less frequently lethal than V583 in the mouse peritonitis model and considerably outcompeted V583 in a murine model of urinary tract infections.
Conclusion: E. faecalis OG1RF carries a number of unique loci compared to V583, but the almost complete lack of mobile genetic elements demonstrates that this is not a defining feature of the species. Additionally, OG1RF's effects in experimental models suggest that mediators of virulence may be diverse between different E. faecalis strains and that virulence is not dependent on the presence of mobile genetic elements.
Figures


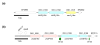


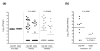
References
-
- Ogier JC, Serror P. Safety assessment of dairy microorganisms: The Enterococcus genus. Int J Food Microbiol. 2007 - PubMed
-
- Paulsen IT, Banerjei L, Myers GS, Nelson KE, Seshadri R, Read TD, Fouts DE, Eisen JA, Gill SR, Heidelberg JF, Tettelin H, Dodson RJ, Umayam L, Brinkac L, Beanan M, Daugherty S, DeBoy RT, Durkin S, Kolonay J, Madupu R, Nelson W, Vamathevan J, Tran B, Upton J, Hansen T, Shetty J, Khouri H, Utterback T, Radune D, Ketchum KA, et al. Role of mobile DNA in the evolution of vancomycin-resistant Enterococcus faecalis. Science. 2003;299:2071–2074. doi: 10.1126/science.1080613. - DOI - PubMed
-
- Domann E, Hain T, Ghai R, Billion A, Kuenne C, Zimmermann K, Chakraborty T. Comparative genomic analysis for the presence of potential enterococcal virulence factors in the probiotic Enterococcus faecalis strain Symbioflor 1. Int J Med Microbiol. 2007;297:533–539. doi: 10.1016/j.ijmm.2007.02.008. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

