Differential activation and trafficking of micro-opioid receptors in brain slices
- PMID: 18612077
- PMCID: PMC3125947
- DOI: 10.1124/mol.108.048512
Differential activation and trafficking of micro-opioid receptors in brain slices
Abstract
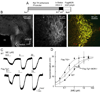
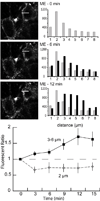
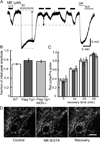
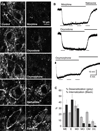
The activation of G protein-coupled receptors results in a cascade of events that include acute signaling, desensitization, and internalization, and it is thought that not all agonists affect each process to the same extent. The early steps in opioid receptor signaling, including desensitization, have been characterized electrophysiologically using brain slice preparations, whereas most previous studies of opioid receptor trafficking have been conducted in heterologous cell models. This study used transgenic mice that express an epitope-tagged (FLAG) micro-opioid receptor (FLAGMOR) targeted to catecholamine neurons by regulatory elements from the tyrosine hydroxylase gene. Brain slices from these mice were used to study tagged MOR receptors in neurons of the locus ceruleus. Activation of the FLAGMOR with [Met5]enkephalin (ME) produced a hyperpolarization that desensitized acutely to the same extent as native MOR in slices from wild-type mice. A series of opioid agonists were then used to study desensitization and receptor trafficking in brain slices, which was monitored with a monoclonal antibody against the FLAG epitope (M1) conjugated to Alexa 594. Three patterns of receptor trafficking and desensitization were observed: 1) ME, etorphine, and methadone resulted in both receptor desensitization and internalization; 2) morphine and oxymorphone caused significant desensitization without evidence for internalization; and 3) oxycodone was ineffective in both processes. These results show that two distinct forms of signaling were differentially engaged depending on the agonist used to activate the receptor, and they support the hypothesis that ligand-specific regulation of opioid receptors occurs in neurons maintained in brain slices from adult animals.
Figures



References
-
- Bohn LM, Gainetdinov RR, Lin FT, Lefkowitz RJ, Caron MG. Mu-opioid receptor desensitization by beta-arrestin-2 determines morphine tolerance but not dependence. Nature. 2000;408(6813):720–723. - PubMed
-
- Bohn LM, Dykstra LA, Lefkowitz RJ, Caron MG, Barak LS. Relative opioid efficacy is determined by the complements of the G protein-coupled receptor desensitization machinery. Mol Pharmacol. 2004;66:106–112. - PubMed
-
- Borgland SL, Connor M, Osborne PB, Furness JB, Christie MJ. Opioid agonists have different efficacy profiles for G protein activation, rapid desensitization, and endocytosis of mu-opioid receptors. J Biol Chem. 2003;278:18776–18784. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

