Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities
- PMID: 18612141
- PMCID: PMC2493614
- DOI: 10.1126/scisignal.127re6
Tissue inhibitors of metalloproteinases in cell signaling: metalloproteinase-independent biological activities
Abstract
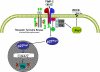
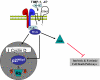
Over the past 20 years, the tissue inhibitors of metalloproteinases (TIMPs) have been implicated in direct regulation of cell growth and apoptosis. However, the mechanisms of these effects have been controversial. Recent work by several laboratories has identified specific signaling pathways and cell surface binding partners for members of the TIMP family. TIMP-2 binding to the integrin alpha(3)beta(1) is the first description of a cell surface receptor for a TIMP family member. TIMP-2 has been shown to induce gene expression, to promote G(1) cell cycle arrest, and to inhibit cell migration. TIMP-1 binding to CD63 inhibits cell growth and apoptosis. These new findings suggest that TIMPs are multifunctional and can act either directly through cell surface receptors or indirectly through modulation of protease activity to direct cell fate. The emerging concept is that TIMPs function in a contextual fashion so that the mechanism of action depends on the tissue microenvironment.
Figures
References
-
- Khokha R, Waterhouse P, Yagel S, Lala PK, Overall CM, Norton G, Denhardt DT. Antisense RNA-induced reduction in murine TIMP levels confers oncogenicity on Swiss 3T3 cells. Science. 1989;243:947–950. - PubMed
-
- Albini A, Melchiori A, Santi L, Liotta LA, Brown PD, Stetler-Stevenson WG. Tumor cell invasion inhibited by TIMP-2. Journal of the National Cancer Institute. 1991;83:775–779. - PubMed
-
- Alvarez OA, Carmichael DF, DeClerck YA. Inhibition of collagenolytic activity and metastasis of tumor cells by a recombinant human tissue inhibitor of metalloproteinases. Journal of the National Cancer Institute. 1990;82:589–595. - PubMed
-
- DeClerck YA, Perez N, Shimada H, Boone TC, Langley KE, Taylor SM. Inhibition of invasion and metastasis in cells transfected with an inhibitor of metalloproteinases. Cancer Research. 1992;52:701–708. - PubMed
-
- Montgomery AM, Mueller BM, Reisfeld RA, Taylor SM, DeClerck YA. Effect of tissue inhibitor of the matrix metalloproteinases-2 expression on the growth and spontaneous metastasis of a human melanoma cell line. Cancer Research. 1994;54:5467–5473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

