Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small-cell lung cancer patients treated with cetuximab and chemotherapy
- PMID: 18612151
- PMCID: PMC3368372
- DOI: 10.1200/JCO.2007.14.0111
Increased EGFR gene copy number detected by fluorescent in situ hybridization predicts outcome in non-small-cell lung cancer patients treated with cetuximab and chemotherapy
Abstract
Purpose: Epidermal growth factor receptor (EGFR) gene copy number detected by fluorescent in situ hybridization (FISH) has proven to be useful for selection of non-small-cell lung cancer (NSCLC) patients for treatment with EGFR tyrosine kinase inhibitors. Here, we evaluate EGFR FISH as a predictive marker in NSCLC patients receiving the EGFR monoclonal antibody inhibitor cetuximab plus chemotherapy.
Patients and methods: Two hundred twenty-nine chemotherapy-naive patients with advanced-stage NSCLC were enrolled onto a phase II selection trial evaluating sequential or concurrent chemotherapy (paclitaxel plus carboplatin) with cetuximab.
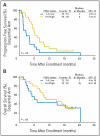
Results: EGFR FISH was assessable in 76 patients with available tumor tissue and classified as positive (four or more gene copies per cell in >/= 40% of the cells or gene amplification) in 59.2%. Response (complete response/partial response) was numerically higher in FISH-positive (45%) versus FISH-negative (26%) patients (P = .14), whereas disease control rate (complete response/partial response plus stable disease) was statistically superior (81% v 55%, respectively; P = .02). Patients with FISH-positive tumors had a median progression-free survival time of 6 months compared with 3 months for FISH-negative patients (P = .0008). Median survival time was 15 months for the FISH-positive group compared with 7 months for patients who were FISH negative. (P = .04). Furthermore, survival favored FISH-positive patients receiving concurrent therapy.
Conclusion: These results are the first to suggest that EGFR FISH is a predictive factor for selection of NSCLC patients for cetuximab plus chemotherapy. Prospective validation of these findings is warranted.
Figures


Comment in
-
Can epidermal growth factor receptor-fluorescent in situ hybridization predict clinical benefit from cetuximab treatment in patients with non-small-cell lung cancer?J Clin Oncol. 2009 Jan 20;27(3):464-5; author reply 465-7. doi: 10.1200/JCO.2008.20.0105. Epub 2008 Dec 8. J Clin Oncol. 2009. PMID: 19064956 No abstract available.
References
-
- Parkin D, Bray F, Ferlay J, et al. Global cancer statistics 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Shepherd F, Rodrigues J, Ciuleanu T, et al. Erlotinib in previously treated non-small cell lung cancer. N Engl J Med. 2005;353:123–132. - PubMed
-
- Herbst RS, Prager D, Hermann R, et al. TRIBUTE: A phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small cell lung cancer. J Clin Oncol. 2005;23:5892–5899. - PubMed
-
- Gatzemeier U, Pluzanska A, Szczesna A, et al. Phase III study of erlotinib in combination with cisplatin and gemcitabine in advanced non-small cell lung cancer: The Tarceva Lung Cancer Investigation Trial. J Clin Oncol. 2007;25:1545–1552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

