Health and economic impact of HPV 16 and 18 vaccination and cervical cancer screening in India
- PMID: 18612311
- PMCID: PMC2480962
- DOI: 10.1038/sj.bjc.6604462
Health and economic impact of HPV 16 and 18 vaccination and cervical cancer screening in India
Abstract
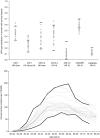
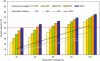
Cervical cancer is a leading cause of cancer death among women in low-income countries, with approximately 25% of cases worldwide occurring in India. We estimated the potential health and economic impact of different cervical cancer prevention strategies. After empirically calibrating a cervical cancer model to country-specific epidemiologic data, we projected cancer incidence, life expectancy, and lifetime costs (I$2005), and calculated incremental cost-effectiveness ratios (I$/YLS) for the following strategies: pre-adolescent vaccination of girls before age 12, screening of women over age 30, and combined vaccination and screening. Screening differed by test (cytology, visual inspection, HPV DNA testing), number of clinical visits (1, 2 or 3), frequency (1 x , 2 x , 3 x per lifetime), and age range (35-45). Vaccine efficacy, coverage, and costs were varied in sensitivity analyses. Assuming 70% coverage, mean reduction in lifetime cancer risk was 44% (range, 28-57%) with HPV 16,18 vaccination alone, and 21-33% with screening three times per lifetime. Combining vaccination and screening three times per lifetime provided a mean reduction of 56% (vaccination plus 3-visit conventional cytology) to 63% (vaccination plus 2-visit HPV DNA testing). At a cost per vaccinated girl of I$10 (per dose cost of $2), pre-adolescent vaccination followed by screening three times per lifetime using either VIA or HPV DNA testing, would be considered cost-effective using the country's per capita gross domestic product (I$3452) as a threshold. In India, if high coverage of pre-adolescent girls with a low-cost HPV vaccine that provides long-term protection is achievable, vaccination followed by screening three times per lifetime is expected to reduce cancer deaths by half, and be cost-effective.
Figures

References
-
- Bhatla N, Ramachandran S, Virmani A, Arora VK, Gulati A, Singla S, Kriplani A, Bell L, Sellors J, Lorincz A, Eder P (2007) Correlation of FastHPV and Cytology: Performance trial results from India. 24th International Papillomavirus Conference and Clinical Workshop, November 3–9 2007, Beijing, China, 1C-03
-
- de Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, Bosch FX (2007) Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis 7: 453–459 - PubMed
-
- Disease Control Priorities Project (DCPP) Available: http://www.dcp2.org/main/Home.html Accessed: 02 October 2007
-
- Drummond MF, Sculpher MJ, Torrance GW, O'Brien BJ, Stoddart GL (eds) (2005) Methods for the Economic Evaluation of Health Care Programs, 3rd edn, New York, NY: Oxford University Press

