Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase
- PMID: 18612415
- PMCID: PMC2440522
- DOI: 10.1371/journal.pone.0002599
Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase
Abstract
Background: There is growing awareness that tumour cells build up a "self-advantageous" microenvironment that reduces effectiveness of anti-tumour immune response. While many different immunosuppressive mechanisms are likely to come into play, recent evidence suggests that extracellular adenosine acting at A2A receptors may have a major role in down-modulating the immune response as cancerous tissues contain elevated levels of adenosine and adenosine break-down products. While there is no doubt that all cells possess plasma membrane adenosine transporters that mediate adenosine uptake and may also allow its release, it is now clear that most of extracellularly-generated adenosine originates from the catabolism of extracellular ATP.
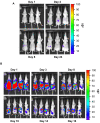
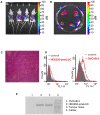
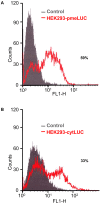
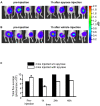
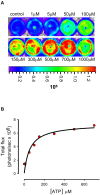
Methodology/principal findings: Measurement of extracellular ATP is generally performed in cell supernatants by HPLC or soluble luciferin-luciferase assay, thus it generally turns out to be laborious and inaccurate. We have engineered a chimeric plasma membrane-targeted luciferase that allows in vivo real-time imaging of extracellular ATP. With this novel probe we have measured the ATP concentration within the tumour microenvironment of several experimentally-induced tumours.
Conclusions/significance: Our results show that ATP in the tumour interstitium is in the hundreds micromolar range, while it is basically undetectable in healthy tissues. Here we show that a chimeric plasma membrane-targeted luciferase allows in vivo detection of high extracellular ATP concentration at tumour sites. On the contrary, tumour-free tissues show undetectable extracellular ATP levels. Extracellular ATP may be crucial for the tumour not only as a stimulus for growth but also as a source of an immunosuppressive agent such as adenosine. Our approach offers a new tool for the investigation of the biochemical composition of tumour milieu and for development of novel therapies based on the modulation of extracellular purine-based signalling.
Conflict of interest statement
Figures


References
-
- Beigi R, Kobatake E, Aizawa M, Dubyak GR. Detection of local ATP release from activated platelets using cell surface-attached firefly luciferase. Am J Physiol Cell Physiol. 1999;276:267–278. - PubMed
-
- Gourine AV, Llaudet E, Dale N, Spyer KM. ATP is a mediator of chemosensory transduction in the central nervous system. Nature. 2005;436:108–111. - PubMed
-
- Albini A, Sporn MB. The tumour microenvironment as a target for chemoprevention. Nat Rev Cancer. 2007;7:139–147. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

