Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51
- PMID: 18612426
- PMCID: PMC2440546
- DOI: 10.1371/journal.pone.0002636
Phase 1 trial of malaria transmission blocking vaccine candidates Pfs25 and Pvs25 formulated with montanide ISA 51
Abstract
Background: Pfs25 and Pvs25, surface proteins of mosquito stage of the malaria parasites P. falciparum and P. vivax, respectively, are leading candidates for vaccines preventing malaria transmission by mosquitoes. This single blinded, dose escalating, controlled Phase 1 study assessed the safety and immunogenicity of recombinant Pfs25 and Pvs25 formulated with Montanide ISA 51, a water-in-oil emulsion.
Methodology/principal findings: The trial was conducted at The Johns Hopkins Center for Immunization Research, Washington DC, USA, between May 16, 2005-April 30, 2007. The trial was designed to enroll 72 healthy male and non-pregnant female volunteers into 1 group to receive adjuvant control and 6 groups to receive escalating doses of the vaccines. Due to unexpected reactogenicity, the vaccination was halted and only 36 volunteers were enrolled into 4 groups: 3 groups of 10 volunteers each were immunized with 5 microg of Pfs25/ISA 51, 5 microg of Pvs25/ISA 51, or 20 microg of Pvs25/ISA 51, respectively. A fourth group of 6 volunteers received adjuvant control (PBS/ISA 51). Frequent local reactogenicity was observed. Systemic adverse events included two cases of erythema nodosum considered to be probably related to the combination of the antigen and the adjuvant. Significant antibody responses were detected in volunteers who completed the lowest scheduled doses of Pfs25/ISA 51. Serum anti-Pfs25 levels correlated with transmission blocking activity.
Conclusion/significance: It is feasible to induce transmission blocking immunity in humans using the Pfs25/ISA 51 vaccine, but these vaccines are unexpectedly reactogenic for further development. This is the first report that the formulation is associated with systemic adverse events including erythema nodosum.
Trial registration: ClinicalTrials.gov NCT00295581.
Conflict of interest statement
Figures

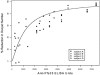
 was used to fit the data, where variables Y and X are the percent reduction of oocyst number, and the ELISA units, respectively, the curve parameters were calculated as a = 1.27 (95% CI 0.96–1.68) and b = 1093 (95% CI 682–1565).
was used to fit the data, where variables Y and X are the percent reduction of oocyst number, and the ELISA units, respectively, the curve parameters were calculated as a = 1.27 (95% CI 0.96–1.68) and b = 1093 (95% CI 682–1565).References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

