The tumorigenicity of mouse embryonic stem cells and in vitro differentiated neuronal cells is controlled by the recipients' immune response
- PMID: 18612432
- PMCID: PMC2440803
- DOI: 10.1371/journal.pone.0002622
The tumorigenicity of mouse embryonic stem cells and in vitro differentiated neuronal cells is controlled by the recipients' immune response
Abstract
Embryonic stem (ES) cells have the potential to differentiate into all cell types and are considered as a valuable source of cells for transplantation therapies. A critical issue, however, is the risk of teratoma formation after transplantation. The effect of the immune response on the tumorigenicity of transplanted cells is poorly understood. We have systematically compared the tumorigenicity of mouse ES cells and in vitro differentiated neuronal cells in various recipients. Subcutaneous injection of 1x10(6) ES or differentiated cells into syngeneic or allogeneic immunodeficient mice resulted in teratomas in about 95% of the recipients. Both cell types did not give rise to tumors in immunocompetent allogeneic mice or xenogeneic rats. However, in 61% of cyclosporine A-treated rats teratomas developed after injection of differentiated cells. Undifferentiated ES cells did not give rise to tumors in these rats. ES cells turned out to be highly susceptible to killing by rat natural killer (NK) cells due to the expression of ligands of the activating NK receptor NKG2D on ES cells. These ligands were down-regulated on differentiated cells. The activity of NK cells which is not suppressed by cyclosporine A might contribute to the prevention of teratomas after injection of ES cells but not after inoculation of differentiated cells. These findings clearly point to the importance of the immune response in this process. Interestingly, the differentiated cells must contain a tumorigenic cell population that is not present among ES cells and which might be resistant to NK cell-mediated killing.
Conflict of interest statement
Figures

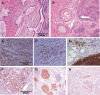
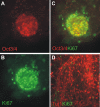
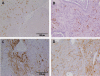
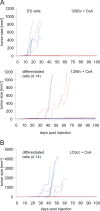


References
-
- Kim JH, Auerbach JM, Rodriguez-Gomez JA, Velasco I, Gavin D, et al. Dopamine neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. - PubMed
-
- Baier PC, Schindehütte J, Thinyane K, Flugge G, Fuchs E, et al. Behavioral changes in unilaterally 6-hydroxy-dopamine lesioned rats after transplantation of differentiated mouse embryonic stem cells without morphological integration. Stem Cells. 2004;22:396–404. - PubMed
-
- Thinyane K, Baier PC, Schindehütte J, Mansouri A, Paulus W, et al. Fate of pre-differentiated mouse embryonic stem cells transplanted in unilaterally 6-hydroxydopamine lesioned rats: histological characterization of the grafted cells. Brain Res. 2005;1045:80–87. - PubMed
-
- Erdö F, Buhrle C, Blunk J, Hoehn M, Xia Y, et al. Host-dependent tumorigenesis of embryonic stem cell transplantation in experimental stroke. J Cereb Blood Flow Metab. 2003;23:780–785. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

